FTCE Chemistry 6-12 Competencies
This competency tests your knowledge of the nature of matter. You can expect to see about 10 multiple-choice questions from this competency.
Let’s explore some specific topics within this competency.
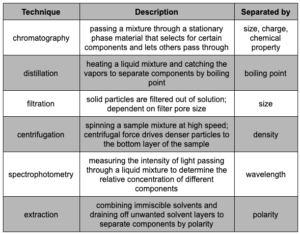
Separating Mixtures
Separating mixtures is a commonly used technique across many fields of laboratory science. To name a few examples: oceanographers filter sediments from seawater, toxicologists extract chemical contaminants from environmental samples, medical technicians centrifuge tissue serums to separate cell types, and chemists distill pure compounds out of crude oil.
Key methods for separating mixtures are explained below.
Molecular Structure of Water
Water is a key component of life on Earth because of its unique physical and chemical properties. A water molecule (H 2
O) is composed of one oxygen atom covalently bonded to two hydrogen atoms.
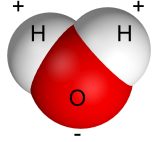
Polarity
Because oxygen is an electronegative atom, it has a strong affinity for electrons. In water, the oxygen atom pulls on the molecular electrons, which gives the O side a partial negative charge and gives the H side a partial positive charge. This means that water is a polar molecule. Nonpolar molecules, such as hydrocarbons and oils, have evenly spaced charges.
Water is sometimes called the “universal solvent” because it is able to dissolve a wide range of substances. Water can dissolve ionic and polar covalent substances such as salts and sugars. However, water cannot dissolve nonpolar substances, which is why oil and water form separate layers after being mixed together.
Hydrogen Bonding
The polar molecular structure of water initiates hydrogen bonding between adjacent water molecules. Hydrogen bonds are strong intermolecular forces between the partial positive charge of the hydrogen side and the partial negative charge on the oxygen side.
Physical properties of water related to hydrogen bonding include:
- surface tension
- adhesion
- cohesion
- high specific heat
- low solid density
Competency 2
This competency tests your knowledge of energy and its interaction with matter. You can expect to see about 14 multiple-choice questions from this competency.
Let’s explore some specific topics within this competency.
Different Forms of Energy
The conservation of energy states that energy cannot be created or destroyed, but it can be transferred between different forms. Different forms of energy include:
- thermal – heat energy generated by a rise in temperature
- electrical – energy generated by the flow ions, an electric current
- light – electromagnetic radiation generated by photons
- chemical – energy stored in molecular and atomic bonds
- nuclear – energy stored in atomic nuclei
- mechanical – energy from an object in motion
Examples of energy transfer:
- Plants convert light energy to chemical energyby making sugars via photosynthesis.
- Animals convert chemical energy to thermal energyby burning fuel from food to keep the body warm.
- Wind turbines convert mechanical energy to electrical energyby channeling the rotation of the blades into an electric current.
Endothermic vs. Exothermic
Chemical reactions generally involve a heat exchange between the reaction components and the environment.
Exothermic reactions
release heat, increasing the temperature of the environment.
Endothermic reactions
absorb heat, decreasing the temperature of the environment.
Whether a reaction is exothermic or endothermic depends on the chemical reaction and the identity of the reagents. In an exothermic reaction, the products are lower energy than the reactants, so the excess energy is released as heat. In an endothermic reaction, the products are higher energy than the reactants, so the extra energy is absorbed as heat.
Kinetic Molecular Theory
Kinetic molecular theory describes the behavior of particles (atoms, molecules, gases) with respect to heat energy in a closed container. It explains the relationships between volume, pressure, kinetic energy, and temperature for ideal gases.
The theory assumes the following:
- Particles are in constant random motion.
- Particles move in straight lines until they collide with another particle or the container wall.
- The volume of the particles is negligible relative to the volume of the container.
- Collisions are completely elastic; there are no attractive or repellent forces.
- Temperature measures the average kinetic energy of the particles.
Competency 3
This competency tests your knowledge of bonding and molecular structure. You can expect to see about 18 multiple-choice questions from this competency.
Let’s explore some specific topics within this competency.
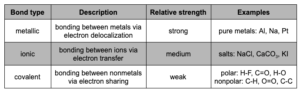
Types of Bonds
Bonds between atoms within a molecule are called intramolecular forces
. The following table summarizes the different types of intramolecular chemical bonds.
Properties of Simple Organic Compounds
Organic compounds are chemical compounds that contain carbon and hydrogen. Organic compounds also commonly contain oxygen, nitrogen, phosphorus, and sulfur and form the basis for the chemistry of living things, or biochemistry.
Well-known organic compounds include:
- carbohydrate sugars, such as glucose (C 6 H 12O 6)
- hydrocarbons, such as propane (C 3H 8)
- amines, such as tryptophan (C 11H 12N 2O 2)
- alcohols, such as ethanol (C 2H 5OH)
Lewis Dot Structure for Molecules
Lewis dot structures are a two-dimensional way to diagram chemical bonds and nonbonded electrons in a molecule using only the outer electrons, or valence electrons.
Lewis dot structures include:
- the chemical symbol of all atoms in the molecule
- lines to denote one pair of bonding electrons
- double lines denote two pairs of bonding electrons
- dots to denote each nonbonded electron
- (e.g., a pair of dots represents a lone pair of electrons)
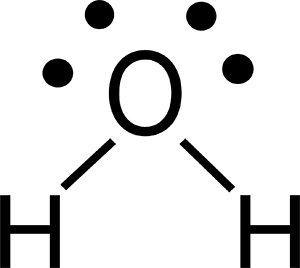
Interpreting the Lewis dot structure for water shows that a central oxygen atom is bonded to two hydrogen atoms with two remaining lone pairs. This structure satisfies the octet rule for oxygen because 2 lone pairs + 2 single bonds = 8 electrons for oxygen’s valence shell.
Naming Basic Chemicals
Chemical nomenclature is standardized internationally by IUPAC (International Union of Pure and Applied Chemistry).
To name chemical compounds, first evaluate the type of compound, then look at the individual atoms.
Ionic compounds
- If anion is polyatomic:
- Write the cation + polyatomic ion with the correct suffix.
- Examples:
- sodium chlorate (NaClO3 )
- calcium carbonate (Ca(CO3)2)
- magnesium sulfite (MgSO3)
- If anion is monoatomic:
- Write the cation + anion + “-ide” suffix.
- Examples:
- sodium chloride (NaCl)
- potassium iodide (KI)
- magnesium oxide (MgO2)
- If the molecule is a covalent compound:
- Write the numerical prefix + “-ide” suffix.
- Examples:
- carbon tetrafluoride (CF4)
- dinitrogen trioxide (N2O3)
- hydrogen sulfide (HS)
- Acids
- If the acid is diatomic:
- Write “hydro-” + anion + “-ic acid” suffix.
- Examples:
- hydrochloric acid (HCl)
- hydroiodic acid (HI)
- hydrobromic acid (HBr)
-
- If the acid is polyatomic and the anion ends in “-ate”:
- Write the anion + “-ic acid” suffix (same as diatomic, but without the “hydro-”)
- Examples:
- chloric acid (HClO3)
- iodic acid (HIO3)
- bromic acid (HBrO3)
-
- If the acid is polyatomic and the anion ends in “-ite”:
- Write the anion + “-ous acid” suffix.
- Examples:
- chlorous acid (HClO2)
- iodous acid (HIO2)
- sulfurous acid (H2SO3)
- Hydroxide bases
- Write the cation + “hydroxide”.
- Examples:
- sodium hydroxide (NaOH)
- potassium hydroxide (KOH)
- calcium hydroxide (Ca(OH2))
Competency 4
This competency tests your knowledge of chemical reactions and stoichiometry. You can expect to see about 21 multiple-choice questions from this competency.
Let’s explore some specific topics within this competency.
Balancing Chemical Reactions
The law of conservation of mass states that mass cannot be created or destroyed. To conserve mass, chemical reactions must be balanced with the same number of each atom in the products and reactants. Coefficients are used to balance chemical reactions. Molecular coefficients differ in meaning from molecular subscripts.
For example:
- 2N ⇒ 2 atoms of nitrogen.
- N 2⇒ 1 molecule of dinitrogen, which is made up of 2 nitrogen atoms connected with a triple bond.
Example 1:
Balance the equation for making carbon dioxide from carbon and oxygen:
C + O → CO 2
The equation is unbalanced because there are 2 atoms of O in the products and only 1 atom of O in the reactants. A coefficient of 2 is added to the reactants side to balance the O atoms.
C +2O → CO 2
Example 2:
Hydrogen peroxide decomposes into water and oxygen. Balance the following equation:
H 2O 2→ H 2O + O 2
In the initial equation there are 2 atoms of O in the reactants and 3 atoms of O in the products. Adding a coefficient to the reactants gives 4H + 4O → 2H + 3O.
2H 2O 2→ H 2O + O 2
Adding a coefficient to water balances the equation with 4H + 4O → 4H + 4O:
2H 2O 2→2H 2O + O 2
Example 3:
The combustion of sucrose in the presence of oxygen produces water and carbon dioxide. Balance the combustion reaction:
C 12H 22O 11+ O 2→ H 2O + CO 2
First balance the C atoms by adding a coefficient of 12 to CO 2:
C 12H 22O 11+ O 2→ H 2O +12CO 2
Next, balance the H atoms by adding a coefficient of 11 to H 2O:
C 12H 22O 11+ O 2→11H 2O + 12CO 2
Finally, add up the number of O atoms on each side: 11+2 → 11+24. Add a coefficient of 12 to O 2
to balance the O atoms to 35 on each side:
C 12H 22O11+12O 2→ 11H O + 12CO 2
Balancing Chemical Reactions – Redox
Oxidation reduction, or redox, reactions are chemical reactions that involve the transfer of electrons between different chemicals.
- When electrons are added, the chemical is reduced, and the ionic charge decreases.
- When electrons are removed, the chemical is oxidized, and the ionic charge increases..
Example 1:
How many electrons are needed to balance the half reaction for the reduction of iron(III) to iron(II)?
Fe 3+ (aq) → Fe 2+ (aq)
Add electrons to the more positive side of the equation, reducing Fe 3+ to balance the charge on both sides:
Fe 3+ (aq) +
e – → Fe 2+ (aq)
Example 2:
Balance the following redox reaction:
Zn (s) + Ni 3+ (aq) → Zn 2+ (aq) + Ni (s)
Split the full reaction into two half reactions:
Reduction:
Ni 3+ (aq) → Ni (s)
Oxidation:
Zn (s) → Zn 2+ (aq)
Add electrons to the more positive side to balance each half reaction:
Reduction:
Ni 3+ (aq) +
3e – → Ni (s)
Oxidation:
Zn (s) → Zn 2+ +
2e – (aq)
Balance the electrons between the two reactions by multiplying a coefficient that makes the electrons equal to the smallest common multiple for the two equations. Since the reduction equation has 3 elections and the oxidation equation has 2, the smallest common multiple is 6.
Reduction:
2 x
(Ni 3+ (aq) + 3e – → Ni (s))
= 2Ni 3+ (aq) +
6e – → 2Ni (s)
Oxidation:
3 x
(Zn (s) → Zn 2+ + 2e – (aq))
= 3Zn (s) → 3 Zn 2+ +
6e – (aq)
Rejoin the half reactions with the new coefficients and divide out the electron term from both sides of the equation:
2Ni 3+ (aq) + 6e
–
→ 2Ni (s) and 3Zn (s) → 3 Zn 2+ + 6e – (aq)
=
3Zn (s) + 2Ni 3+ (aq) +
6e – → 2Ni (s) + 3 Zn 2+ (aq) +
6e – =
3Zn (s) + 2Ni 3+ (aq) → 2Ni (s) + 3 Zn 2+ (aq)
Example 3:
Balance the following redox reaction in an acidic solution:
Cr 2
O 7 2- (aq) + Co 2+ (aq) → Cr 3+ (aq) + Co 3+ (aq)
Split the full reaction into two half reactions. Cobalt is being oxidized from a charge of +2 to +3 and chromium is being reduced from a charge of +6 to +3. Balance elements other than O and H:
Reduction:
Cr 2
O 7 2- (aq) → 2Cr 3+ (aq)
Oxidation:
Co 2+ (aq) → Co 3+ (aq)
Add water molecules (H 2
O) and hydrogen ions (H + ) to obey the law of conservation of mass for the reduction equation:
Reduction:
14H+(aq)+ Cr 2O 7 2- (aq) → 2Cr 3+ (aq) +7H2O(l)
Add electrons to the more positive side to balance each half reaction:
Reduction:
6e – + 14H + (aq) + Cr 2
O 7 2- (aq) → Cr 3+ (aq) + 7H 2
O(l)
Oxidation:
Co 2+ (aq) → Co 3+ (aq ) +
e – Balance the electrons between the two reactions by multiplying a coefficient that makes the electrons equal to the smallest common multiple for the two equations. Since the reduction equation has 6 electrons and the oxidation equation has 1, the smallest common multiple is 6:
Reduction:
1 x(6e – + 14H + (aq) + Cr 2O 7 2- (aq) → Cr 3+ (aq) + 7H 2O(l))=6e–+ 14H + (aq) + Cr 2
O 7 2- (aq) → Cr 3+ (aq) + 7H 2O(l)
Oxidation:
6 x(Co2+(aq) → Co3+(aq )+ e – ))= 6Co2+(aq) → 6Co3+(aq) +6e –
Rejoin the half reactions with the new coefficients and divide out the electron term from both sides of the equation:
6e–+ 14H + (aq) + Cr 2O 7 2- (aq) + 6Co 2+ (aq) → Cr 3+ (aq) + 7H 2O(l) + 6Co 3+ (aq) +
6e-=
14H + (aq) + Cr 2
O 7 2- (aq) + 6Co 2+ (aq) → Cr 3+ (aq) + 7H 2
O(l) + 6Co 3+ (aq)
Solve Mass-Gas Stoichiometry Problems
The ideal gas law is represented by this equation:
pV = nRT
p ⇒ pressure (atm)
V ⇒ volume (L)
n ⇒ amount of gas (moles)
R ⇒ ideal gas constant (0.08205 L · atm mol -1 K -1 )
T ⇒ temperature (K)
Relationships derived from the ideal gas law can be used to calculate parameters in a chemical reaction involving gases in a closed container. Additionally, at standard temperature and pressure conditions (273K, 1 atm), 1 mole of gas fills a volume of 22.4 L.
Example 1:
Given the following chemical equation, what volume of methane (CH 4 ) is produced by a reaction of 2 moles of hydrogen gas (H 2 ) with an excess of carbon dioxide (CO 2 ) at STP?
CO 2
(g) + 4H 2
(g) → CH 4
(g) + 2H 2
O (g)
Use dimensional analysis and stoichiometry to solve.
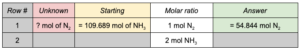
- Write the unknown: volume of CH 4.
- Find the starting point, or the known value: 2 mol of H 2.
- Use the molar ratio from the chemical equation to convert moles of H 2to moles of CH 4.
- Use the STP conversion factor to convert moles of CH 4to liters of CH 4.
- Multiply values in Row 1 (numerator) and divide by values in Row 2 (denominator) to find the final answer.

Example 2:
Nitrogen gas (N 2 ) reacts with hydrogen gas (H 2 ) to form ammonia (NH 3
). The product of the reaction is siphoned into a separate container with a volume of 30L and the whole system is kept at a constant 300 K. Given the following chemical equation, how many moles of N 2
must react with an excess amount of H 2
to raise the pressure of NH 3
in the collection vessel from 10 atm to 100 atm?
N 2
(g) + 3H 2
(g) → 2NH 3
(g)
Write down the known and unknowns:
P NH3
= 90 atm
V NH3
= 30 L
n NH3
= ?
nN 2
= ?
R = 0.08205 L · atm mol -1 K -1 T = 300K
Use the ideal gas law to find the moles of NH 3
that need to be produced to raise the pressure by 90 atm:
PV = nRT
Solve for moles (n) and substitute in known values:
⇒ n = PV/RT
n = 90 atm x 30 L / (300 K x 0.08205 L · atm mol -1 K -1 )
= 109.689 moles NH 3
Use dimensional analysis and stoichiometry to find the moles of N 2
needed to produce 109.689 moles of NH 3
:
Chemical Reaction – Double Replacement
Double replacement or double displacement reactions are chemical reactions in this form:
AB + CD → AC + BD
Component ions of the reactants switch places to form the products. Double replacement reactions are usually driven by the precipitation of a solid in an aqueous solution.
Example 1:
Balance the following double replacement reaction for the formation of solid silver sulfate. Include a net ionic equation:
Ag(NO 3 ) 2
(aq) + CaSO 4
(aq) → AgSO 4
(s) + Ca(NO 3 ) 2
(aq)
Balance chemicals to obey the law of conservation of mass. All species are already balanced:
Ag(NO 3 ) 2
(aq) + CaSO 4
(aq) → AgSO 4
(s) + Ca(NO 3 ) 2
(aq)
Write the full ionic equation by splitting up ionic compounds:
Ag 2+ (aq) + 2NO 3 – (aq) + Ca 2+ (aq) + SO 4 2- (aq) → AgSO 4
(s) + Ca 2+ (aq) + 2NO 3 – (aq)
Identify and divide out spectator ions that are not involved in formation of the solid product. This is the net ionic equation.
Ag 2+ (aq) +
2NO
3
–
(aq)
+
Ca 2+ (aq)
+ SO 4 2- (aq) → AgSO 4
(s) +
Ca
2+
(aq)
+
2NO
3
–
(aq)
= Ag 2+ (aq) + SO 4 2- (aq) → AgSO 4
(s)
Example 2:
Predict the products for the following double displacement reaction. Write the balanced equation and find the net ionic equation:
MgCl 2
(aq) + NaOH (aq) → ???
Predict the products by switching the ions in the reacting compounds. Solid magnesium hydroxide is formed:
MgCl 2
(aq) + NaOH (aq) → Mg(OH) 2
(s) + NaCl (aq)
Balance chemicals to obey the law of conservation of mass by adding coefficients:
MgCl 2
(aq) +
2
NaOH (aq) → Mg(OH) 2
(s) +
2
NaCl (aq)
Write the full ionic equation by splitting up ionic compounds:
Mg2+ (aq) + 2Cl– (aq) + 2Na+ (aq) + 2OH– (aq) → Mg(OH)2 (s) + 2Na+(aq) + 2Cl–(aq)
Identify and divide out spectator ions that are not involved in formation of the solid product. This is the net ionic equation:
Mg 2+
(aq) +
2Cl
–
(aq)
+
2Na + (aq)
+ 2OH – (aq) → Mg(OH) 2
(s) +
2Na
+
(aq)
+
2Cl
–
(aq)
=
Mg 2+ (aq) + 2OH – (aq) → Mg(OH) 2
(s)
Competency 5
This competency tests your knowledge of atomic theory and structure. You can expect to see about 12 multiple-choice questions from this competency.
Let’s explore some specific topics within this competency.
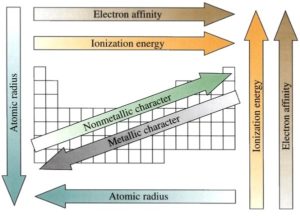
Physical Properties of the Periodic Table
The periodic table has trends for different atomic properties. Key periodic trends are defined
below.
- Ionization energy – the energy required to remove an electron from an atom. Ionization energy is the highest for small nonmetals because the electrons are closer to the nucleus and the attraction force is stronger.
- Electron affinity – the energy released when an electron is added to an atom. Electron affinity is highest for halogens and smaller atoms do not have full octet.
- Atomic radius – the distance from the center of the nucleus to the outermost electrons in an atom. Atomic radius increases with atomic number because more subatomic particles take up more space.
- Metallic character – how easily electrons are released from an atom. Metallic character increases with atomic number because larger atoms have a weaker attraction between the nucleus and the electrons
Nuclear Energy: Fission vs. Fusion
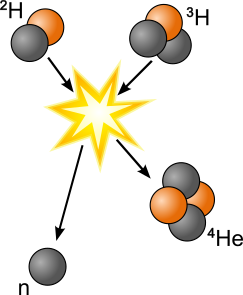
In nuclear fusion,
small atomic nuclei are combined to create one larger nucleus. Generally, nuclear fusion reactions release neutrons; however, only lighter fusion products are exothermic. Heavy fusion products are endothermic. Solar energy comes from fusion reactions between hydrogen nuclei in the sun’s core.
In nuclear fission,
large atomic nuclei are split into two or more fragmented nuclei. Generally, nuclear fission also releases extra neutrons, heat energy, and gamma radiation. Power from nuclear fission can be used to generate electricity.
Competency 6
This competency tests your knowledge of the nature of science. You can expect to see about 10 multiple-choice questions from this competency.
Let’s explore some specific topics within this competency.
Scientific Inquiry
Scientific inquiry includes a series of steps that form the foundation for effective problem-solving across all fields of science.
The scientific method is summarized in the table below:
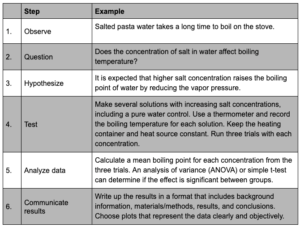
Scientific inquiry
differs from nonscientific inquiry in that it aims to be systematic, unbiased, and quantitative. It is a form of active learning in which participants engage with each step of the scientific method. Non-scientific inquiry includes inductive reasoning and passive learning through lectures and readings. Inductive reasoning builds conclusions based on previous experiences, whereas learning from lectures relies on an authority figure to disseminate the information through a lecture or textbook.
Independent vs. Dependent Variables
Experiments use variables to test the effect of [A] on [B]. How does volume affect pressure for a gaseous mixture? Does hydrocarbon saturation affect the freezing point of a lipid? What is the effect of temperature on the solubility of sodium salts in water?
The independent variable is the parameter that causes an effect [A].
The dependent variable is the parameter being measured [B]. This is also called the response variable.
Competency 7
This competency tests your knowledge of measurement. You can expect to see about 10 multiple-choice questions from this competency.
Let’s explore some specific topics within this competency.
Precision vs. Accuracy
Accuracy
refers to how close a measurement is to the true value. Accuracy gives information about the correctness of the data.
Precision
refers to how close a group of measurements are to each other. Precision gives information about the consistency of the data.
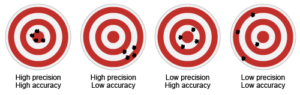
- Target 1 shows both high precision and high accuracy because all of the marks are clustered near the bullseye.
- Target 2 shows high precision and low accuracy because all the marks are clustered near each other but not near the bullseye.
- Target 3 shows low precision and high accuracy because the marks are not clustered together, but individually they are each close to the bullseye.
- Target 4 shows low precision and low accuracy because the marks are not clustered together and they are not close to the bullseye
Scientific Notation
Scientific notation is an abbreviated way to represent numbers that are very large and very small by using powers of 10. For example, 2000 is the same as 2 x 1000, which is the same as 2 x 10 3 . Scientific notation can use ‘E’ instead of ‘10’ (2 x 10 3 is the same as 2E+3)
Example 1:
Write 0.000000000000045 in scientific notation.
Answer:
4.5 x 10 -14 Each decimal position changes the scientific notation by a factor of 10. Since the decimal is moving to the right, add 10 -1 for each increment of 10.
Example 2:
Write 3920000000000000000000 in scientific notation.
3.92 x 10 21 Each decimal position changes the scientific notation by a factor of 10. Since the decimal is moving to the left, add 10 1 for each increment of 10.
Competency 8
This competency tests your knowledge of appropriate laboratory use and procedures. You can expect to see about five multiple-choice questions from this competency.
Let’s explore one topic from this competency.
Stay Safe in Labs
Safety is the key objective of any lab. Attention to safety is particularly important in chemistry, where hazards include toxic and corrosive chemicals, explosive reactions, broken glassware, and open flames.
General safety practices that should be reviewed regularly in a chemistry lab include:
- Always wear appropriate clothing, including long pants and closed-toe shoes.
- Always wear personal protective equipment (PPE), including goggles, lab coat, and gloves.
- Never eat, drink, or store food in the lab.
- Dispose of excess chemicals in the appropriately labeled waste container.
- Review the location and operation of safety equipment, including fire extinguishers, eye washes, and emergency showers.
- Open chemical containers away from faces, preferably under a fume hood.
- Never use glassware that is chipped, cracked, or broken. Dispose of in designated glass waste or sharps containers.
- Never taste or smell chemicals; instead, waft the air toward the face to sense odors.
- Always dilute acids by slowly adding concentrated acid to a container of water rather than adding water to a container of concentrated acid.
- Keep electrical outlets from getting wet or being overloaded
- Always notify a teacher immediately if there is a chemical spill or lab accident.
- Always wash hands with soap and water before leaving the lab or touching any personal items.
Other rules for standard chemical practice:
- Avoid cross-contamination by using clean utensils and never putting excess chemicals back in the original container.
- Read the volume of a graduated cylinder from the lowest level of the liquid line, or the meniscus.
- Use calibrated equipment (graduated cylinders, volumetric flasks, pipettes) for measuring and non-calibrated equipment (beakers, Erlenmeyer flasks) for mixing.
And that’s some basic information about the FTCE Chemistry 6-12 test.
