The Praxis 5435 exam has been retired and replaced by the Praxis 5436 exam. To prepare for the Praxis General Science (5436) exam use our updated Praxis 5436 practice test!
Welcome to our free Praxis®️ General Science: Content Knowledge practice test and prep page. We’ll be introducing you to the key concepts you need to know to pass this exam. This is one of the free resources we provide so you can see how prepared you are to take the official Praxis®️ 5435 Test.
While this free guide outlines the content categories found on the exam, our paid Praxis®️ General Science: Content Knowledge Study Guide covers EVERY concept you need to know and is set up to ensure your success! Our online General Science: Content Knowledge study guide provides test-aligned study material using interactive aids, videos, flash cards, quizzes and practice tests.
Will I pass using this free article? Will I pass using your paid study guide?
If you use this guide and research the key concepts on the General Science: Content Knowledge exam on your own, it’s possible you will pass, but why take that chance? With our paid study guide, we guarantee you will pass.
Not ready to start studying yet? That’s OK. Keep reading, and when you’re ready, take our free Praxis®️ General Science: Content Knowledge practice tests.
In this article, we will cover:
Praxis®️ General Science Test Information

Overview
The Praxis®️ General Science: Content Knowledge exam is required for candidates planning to teach high school science. It is one of many prerequisites for state licensing programs. This test is designed to ensure that potential teachers have sufficient knowledge needed to teach science at the higher levels. Candidates who take this test typically have a bachelor’s degree that has included some science and education.
Format:
There are 135 selected-response questions. The questions are taken on a computer in a multiple-choice format.
Cost:
$130
Scoring:
Passing scores vary according to the state or agency you’re taking the exam through. Passing scores for the Praxis®️ General Science exam range from 143-160 depending on your state. Verify your passing scores by test or state at: https://www.ets.org/praxis/al/test-takers/plan-your-test/score-requirements.html
Pass rate:
Pass rate percentages vary according to each state’s passing scores but the average performance range on the Praxis General Science: Content Knowledge exam is 158-172.
Study time:
The amount of study time you need will depend on many factors, including your educational history, subject background, and how comfortable you are with a test-taking environment. In general, we recommend people spend 1 to 3 months studying.
What test takers wish they would’ve known:
- Take several practice tests to get an idea of the format
- Create a study plan
- Keep track of time during the test
- Create a pacing plan
Information and screenshots obtained from the ETS Praxis®️ website: https://www.ets.org/praxis/prepare/materials/5435
Praxis®️ General Science: Scientific Methodology, Techniques, and History
Overview
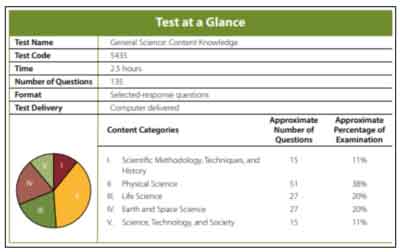
The Scientific Methodology, Techniques, and History content category has about 15 multiple-choice questions, which account for about 11% of the Praxis®️ General Science test.
This part has seven sections:
- Methods of Scientific Inquiry and Design
- Processes Involved in Scientific Data Collection and Manipulation
- Interpret and Draw Conclusions from Data Presented in Tables, Graphs, Maps, and Charts
- Procedures for Correct Preparation, Storage, Use, and Disposal of Laboratory Materials
- How to Use Standard Equipment in the Laboratory and the Field
- Safety and Emergency Procedures in the Laboratory
- Major Historical Developments of Science
So, let’s start with Methods of Scientific Inquiry and Design.
Methods of Scientific Inquiry and Design

This section tests your knowledge on the scientific experiment process.
Let’s discuss a concept that will more than likely appear on the test.
Experimental Design
An experimental design is used to minimize the amount of error and maximize the control you have over your experiment. An experimental design ensures that you are testing a single question (hypothesis), you are testing only a single variable at a time, and you repeat the experiment enough times to gather meaningful results. It is the core of the scientific process. The goal is to provide you with solid, factual evidence.
An experimental design is made of several components:
Hypothesis: The question you are trying to answer. This can be either open-ended or in yes/no format, but must clearly address your problem statement.
Independent Variables: The variables (things) that are changed in order to test the effects on the dependent variable.
Dependent Variables: The variable being tested or measured in the experiment. It is called dependent because it “depends” on the independent variable. Examples: If you are testing how height changes for different ages, how tall you are is the dependent variable and age is the independent variable.
Controls: These are variables that do not change during the experiment. They serve as the thing to which you compare your results.
Sources of Error: It is important to identify any ways the experiment may lead to false results. Sources of error can be human error (you recorded data incorrectly or you didn’t clean your instruments after each repetition) or error in the way you created your experiment (too many variables).
Processes Involved in Scientific Data Collection and Manipulation
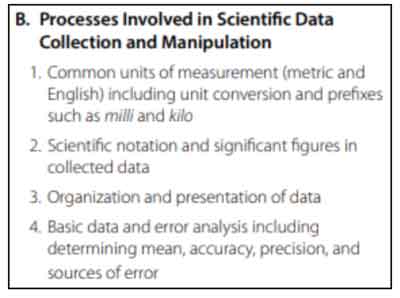
This section tests your knowledge on how to process, collect, and enter scientific data. You’ll review basic units of measurement (with conversions), scientific notation, and how to conduct error analysis.
Interpret and Draw Conclusions from Data Presented in Tables, Graphs, Maps, and Charts
This section tests your knowledge of interpreting data through tables and graphs. You’ll review how to identify trends in data, relationships between variables, identify predictions or outliers, and draw conclusions from your data.
Procedures for Correct Preparation, Storage, Use, and Disposal of Laboratory Materials

This section tests your knowledge on proper laboratory protocol, including safety, types of equipment, handling procedures, storage, maintenance, and labeling.
Here’s a concept likely to appear on the test.
Preparation of Materials
You prepare a solution by dissolving a set amount of the solute (a solid) into a specific amount of the solvent (the liquid you’re dissolving into). Concentration of solutions is usually expressed in moles (M) per liter.
Staining slides is used to help us see the results of our experiments better at the microscopic level. To create a stain: Collect a drop of the stain using an eyedropper or a pipette. Place a drop of the stain onto your cover slide. The stain should cover your specimen. If not, add another drop. Then place the slide on the viewing tray of the microscope. Clean the slide and coverslip when you are finished.
To label your slide: Remove it from the microscope and place it in a storage container with a clear label.
For an example of a staining experiment and labeling, check out: https://www.youtube.com/watch?v=dxv4M4HHUgs
How to Use Standard Equipment in the Laboratory and the Field
This section tests your knowledge on proper use, storage, and maintenance of laboratory instruments.
Let’s talk about a concept that is expected to come up on the test.
Appropriate Storage
pH probes (also known as pH electrodes or pH meters) measure voltage or acidity produced by a solution. You want to make sure you always keep the pH probe moist. Store it in a solution of 4 M KCl (if that is not available, use a pH 4 or 7 buffer solution). You should never store your pH probe in distilled or deionized water, as that can cause damage and render your probe useless.
Electrodes must be rinsed between samples with distilled water. After storage, you may notice that little crystals have formed on the bulb. Simply rinse it and blot it dry before use, as this is normal.
Safety and Emergency Procedures in the Laboratory
This section tests your knowledge on safety in a science laboratory and properly handling emergency situations.
Let’s discuss a concept likely to pop up on the test.
Chemical Spills
A chemical spill can occur when the chemicals, compounds, or solutions accidentally spill from the testing material onto a surface. Spills can happen on tables or get onto people’s skin or clothes. In the event of a spill, take the following steps:
-
- Prevent the further spread of the spill.
- Neutralize acids and bases if possible.
- Control the spread of the liquid and absorb it using disposable gloves and absorbent paper.
- Collect and use clean-up materials.
- Dispose of wastes appropriately. Put contaminated gloves, lab coats, or materials in a bag.
- Decontaminate the area or affected equipment.
- Refer the student to the eye-wash station or hand-wash station if applicable.
Major Historical Developments of Science
This section tests your knowledge of major developments in science, including theories and figures.
This is a concept likely to come up on the test.
Atomic Theory
Atomic theory is a five-point principle that explains chemistry and the composition of matter at a very small level: atoms. The concept of “atoms” was first suggested by the Ancient Greeks, but scientists didn’t have the tools strong enough to prove this until the Scientific Revolution in the 1700 and 1800s. In 1803, John Dalton developed the “atomic theory.” This had a tremendous impact on science because this marks the beginning of our understanding of chemistry and the composition of matter.
In 1803, John Dalton developed the “atomic theory.” This contains five principles that guided scientific understanding. We still use these concepts today:
- All matter consists of tiny particles, called atoms.
- Atoms can’t be created, destroyed, or divided into smaller pieces. This is based on the law of conservation of mass, which states that mass cannot be created or destroyed in a chemical reaction. Similarly, atoms can’t be created or destroyed.
- Atoms of a given element are identical in size and mass. Atoms from different elements differ in size and mass. Atoms of the same element have all identical weights. Every atom in oxygen is identical to every other oxygen atom. Atoms of different elements have different weights: that is, an oxygen atom is different from a mercury atom.
- In chemical reactions, atoms combine in small, whole-number ratios. This is important for chemical reactions, and means that the ratio of atoms is precise. A reaction can’t end with half an atom or 3/8 of an atom. It’s a whole number or a whole ratio.
- In chemical reactions, atoms are combined, separated, or rearranged. This is always in a whole number or whole-number ratio.
And that’s some basic info about the Scientific Methodology, Techniques, and History content category of the Praxis®️ General Science exam.
Praxis®️ General Science: Physical Science
Overview
The Physical Science content category has about 51 multiple-choice questions, which account for about 38% of the test.
This part has three sections:
- Basic Principles
- Chemistry
- Physics
So, let’s start with the Basic Principles.
Basic Principles
This section tests your knowledge on the relationship between energy and matter. You’ll review three laws of thermodynamics, kinetic vs. potential energy, physical and chemical property changes, and temperature scales.
Let’s discuss some concepts that will more than likely appear on the test.
Laws of Thermodynamics
Thermodynamics is a branch of physical science. Specifically, thermodynamics refers to the relationship between heat and temperature in relation to energy and work, such as in a chemical reaction. There are 4 laws of thermodynamics with which you need to be familiar:
- The first law of thermodynamics is the conservation of energy law. This states that in a reaction, energy cannot be created or destroyed. However, energy can be transferred in a reaction or converted to another form of energy. Energy in a system (or even the universe) is constant. Heat can flow into a system or out of it, but it is all at the expense of energy and work. For example, think about a generator. The amount of energy you input (fuel) is the same amount of thermal energy (heat) that will be emitted. Heat cannot be randomly created or destroyed.
- The second law of thermodynamics is about entropy and equilibrium. Entropy refers to disorder.
This law states that when energy changes form or when matter moves freely, entropy (disorder) increases. This is because closed systems always gravitate toward “thermodynamic equilibrium,” or entropy. While the 1st law is about the total amount of energy in a system, the 2nd law is about the quality of energy. As energy is transferred, more of it is wasted.
Think of the second law like a messy room. If the room is not kept clean, it will become messier over time. When the room is cleaned, disorder (entropy) decreases, but you had to put a lot of effort (work) into cleaning it, resulting in an increase in entropy outside of the room.
Kinetic and Potential Energy
Energy is the ability to do work. Objects can have stored energy (potential energy), when work has been or will be done. Objects can also have kinetic energy, which is the energy force when the object starts to move. When you stretch a rubber band, it has potential energy. That energy turns into kinetic energy when you release the rubber band and launch it into the air.
Potential energy is stored so that it can later become kinetic. The word “potential” helps clue you in that this energy is stored and not yet used. It has the potential to be used for something else. Potential energy can be electrical, chemical, or nuclear energy, and, in chemistry, is measured in Joules (abbreviated as J). Think about a ball that has been placed at the top of a hill. This ball has stored energy (potential energy) that has not been used yet.
Kinetic energy is the energy released from an object moving. This is the actual motion or action of the object. Kinetic energy can be an object moving from place to place, being rotated, or vibrated. Kinetic energy is also measured in Joules (J). If we think about a ball at the top of a hill, kinetic energy is the energy released when it rolls down.
Chemistry

This section tests your knowledge of chemical reactions, types of reactions, and how to write formulas. You will also review trends of elements based on the periodic table, and understand the properties of metals, nonmetals, and noble gases.
Let’s dive into some concepts that are likely to appear on the test.
Chemical Reactivity
A chemical reaction is a process where one or more substances are changed into a new substance. Chemical reactions are based on changes that involve the positions of electrons, forming or breaking bonds with atoms.
In a chemical reaction, you have a reactant (or substrate), which is the compound undergoing a change. The reagent is the partner of the reactant. The product is the end result of the reaction. Chemical reactions are written as equations:

We say that the reactants yield a product, indicated by the arrow sign.
The periodic table organizes elements by their properties; notably, their electronic structure (valence electron configuration).
Metals are solid, shiny, and good conductors of both electricity and heat. They have a very high melting temperature. Metals can be molded into thin wires (they are ductile), and can be easily hammered into thin sheets (they are malleable). Metals tend to lose electrons easily in reactions. Most elements are metals, and these elements make up the bulk of the periodic table, starting on the left. Examples: Gold, aluminum, copper, iron, lead, tin, and silver are all metals.
Nonmetals are on the right side of the periodic table. Nonmetals have the opposite properties as metals. They are dull, cannot be molded easily (they are not ductile) and they cannot be hammered into sheets (they are not malleable). They are poor conductors of heat and electricity. Nonmetals tend to gain electrons in chemical reactions. Examples: oxygen, carbon, hydrogen, sulfur, and iodine are some nonmetals.
Metalloids are elements that border the stair-stepped line across the periodic table. They are also known as semimetals. They have properties that are in between metals and nonmetals. They can conduct electricity, but not very well. Metalloids can be shiny or dull, and their shape can be changed.
Noble gases: There are only 4 noble gases: argon, xenon, helium, and neon. These have the maximum number of valence electrons (full outer shell of electrons), which means they are non-reactive. Noble gases don’t really take part in chemical reactions. Noble gases never bond to other elements in nature.
Chemical Formulas
A compound is a molecule that contains at least two different elements.
A chemical compound is where two (or more) substances of different elements are bonded together. Water (H20: two hydrogen and one oxygen atom), carbon dioxide (CO2: one carbon atom and two oxygen atoms bonded together), glucose (C6H12O6: six carbon, 12 hydrogen, and six oxygen atoms), and sodium chloride (NaCl: one sodium atom and one chloride atom) are all chemical compounds, because they contain different substances bonded together.
A chemical formula shows the symbols of the elements, and the subscript refers to the number of atoms of that element. When combined, these substances create a compound.
Elements are written in shorthand using capital letters and subscripts for number of atoms. Here are some examples of chemical formulas:
Na: Sodium
H20: Water
C6H12O6: Glucose
NaCl: Table salt
Physics

This section tests your knowledge on physical properties of motion and sound.
Let’s talk about some concepts bound to pop up on the test.
Laws of Motion
Sir Isaac Newton developed three laws of motion to describe how bodies interact. These may seem obvious today, but his laws set the foundation for modern physics. Remember that a force is a push or pull on an object, and external forces are any outside forces that act on something. We measure the force in Newtons (N). Friction causes objects to slow down, and inertia is the tendency of an object to remain at rest or in motion. Equilibrium occurs when the forces on the system are balanced.
Newton’s First Law: This law is also known as the law of inertia. This law states that an object at rest will stay at rest until a force acts on it. Similarly, an object in motion stays in motion unless an external force acts on it. This means that things can’t stop or start randomly by themselves. It takes a force to change it. If you slide a hockey puck across the ice, it will eventually stop because of the friction of the ice against the puck. The puck doesn’t just randomly start sliding. You had to initiate the sliding.
Newton’s Second Law: This law explains that when a constant force acts on a body, it causes the body to accelerate and change its velocity at a constant rate. The force acting on an object is equal to the mass of the object times its acceleration. This is often written as the formula: F= m *a, where F= force, m = mass, and a = acceleration. The more mass an object has, the more force you need to move it. For example, it’s easier to push an empty shopping cart than a full one. The full cart has more mass and requires more force to move it.
Newton’s Third Law: For every action (force), there is an equal and opposite reaction. Forces always occur in pairs. When a body pushes against another, the second body pushes back with equal amount of force. When you’re on a skateboard and you push backward, the same amount of force drives the skateboard forward. When you throw a ball to the ground, it bounces back with equal amount of force.
Waves
A wave is any transfer of energy from one point to another without a transfer of material. A wave is a disturbance that travels through a medium, transporting energy without transporting the matter. A wave can be an ocean wave, but we can also think of sound waves, where vibrations of air molecules carry energy from one place to another. When drawing a wave on a graph, the highest point of the wave is called the crest, and the lowest point is called the trough.
We can explain waves based on their characteristics. Waves can be transverse or longitudinal.
Transverse waves occur when the particles move in a direction perpendicular to the direction the wave moves. When you picture the vibration of strings, ripples on water surface, an audience doing the “wave,” or light waves, these are all transverse waves.
Longitudinal waves occur when the particles move parallel to the direction of the wave. An ocean wave, ripples from a stone across a pond, and sound waves are all longitudinal.
There are several words we use to describe the qualities of a wave:
Frequency: The number of times per second that the wave cycles. Frequency is measured in Hertz, or the letter (f).
- Amplitude: This is measured by the difference in the height of the wave (crest) and the wave at resting position. This measures the displacement of the wave from rest, and measures the overall intensity. When thinking about sound waves, the amplitude measures the loudness of the sound.
- Wavelength: The distance between two wave cycles. This is measured by the distance between two crests or two troughs (two high points or two low points). Wavelength is represented by the Greek letter lambda (λ).
- Speed: Speed measures how fast the wave is moving. This is the horizontal speed of a point on the wave, measured in meters per second (m/s).
- Energy: Waves transfer energy without matter.
And that’s some basic info about the Physical Science content category of the Praxis®️ General Science exam.
Praxis®️ General Science: Life Science
Overview
The Life Science content category has about 27 multiple-choice questions, which account for about 20% of the test.
This part has nine sections:
- Basic Structure and Function of Cells and Their Organelles
- Key Aspects of Cell Reproduction and Division
- Basic Biochemistry of Life
- Basic Genetics
- Theory and Key Mechanisms of Evolution
- Hierarchical Classification Scheme
- Major Structures of Plants and Their Functions
- Basic Anatomy and Physiology of Animals, including the Human Body
- Key Aspects of Ecology
So, let’s start with Basic Structure and Function of Cells and Their Organelles.
Basic Structure and Function of Cells and Their Organelles
This section tests your knowledge of cell biology, structure, and the cell cycle.
Let’s discuss a concept that will more than likely appear on the test.
Cell Membranes
All organisms are made of one or more cells, and cells carry out specific functions. The main parts of a cell are plasma membrane (also called cell membrane), cytoplasm, and nucleus.
The cell membrane surrounds the entire cell, like a casing. In animal cells, this is a cell membrane; in plant cells, it is the rigid cell wall. The cell membrane is made up of a phospholipid bilayer. Phospholipids are fatty acids arranged in two layers. Each molecule of phospholipids has a “head” and a “tail.” The head is hydrophilic, which means it is attracted to water. The tail is hydrophobic, which means it repels water. This is important to remember it changes how the cell membrane allows things to pass through. Molecules that are hydrophobic can pass through the plasma membrane if they are small enough, and molecules that are hydrophilic cannot easily pass through.
The cell membrane controls what goes in and out of the cell. It does this through either active or passive transport. Both methods use ion channels to move ions across the cell membrane.
Active transport requires energy and work. Active transport moves ions from low concentration to high, using ATP.
Passive transport does not require energy or work. Passive transport moves ions from high concentration to low, using no metabolic energy (no ATP). This is the simplest form of energy transport across the cell membrane.
Key Aspects of Cell Reproduction and Division
This section tests your knowledge on the cell cycle and reproductive processes.
This is a concept that is likely to appear on the test.
Cell Reproduction and Division
The cell cycle refers to the stages that a cell undergoes between its formation (birth) and reproduction.
The cell cycle begins in interphase, the period of cell growth and protein synthesis. Interphase has three stages: G1, S, and G2. In G1 (also known as “gap one,” or “first gap”), the cell is accumulating the building blocks (chromosomal DNA) that it will need for reproduction. Think of this phase as cell growth.
In S phase, the cell begins DNA synthesis (S phase = synthesis phase). Here, DNA replication begins and creates two identical copies of chromosomes, called “sister chromatids.” Later on, these will be important for the division.
Next is the G2 phase, or second gap. Here, the cell replenishes its energy and begins synthesizing proteins that will be necessary for mitosis. In G2, there is more cell growth.
The mitotic phase is next. This process takes the duplicated daughter cells (from G1), and aligns, separates, divides them again. The cell is divided into identical daughter cells in this phase. Mitosis is the process of the cell dividing the nucleus, and cytokinesis (the final portion of mitosis), is where the cytoplasm physically separates into two daughter cells.
Mitosis is divided into five phases: prophase, prometaphase, metaphase, anaphase, and telophase.
- Prophase condenses chromosomes, and spindle fibers emerge. The nuclear envelope begins to break down. Centrosomes move toward opposite poles.
- Prometaphase continues these processes, and the nuclear envelope begins to disappear.
- In metaphase, all of the chromosomes are aligned between the two poles of the cell.
- Anaphase is when the cell becomes visibly elongated.
- Telophase is when the chromosomes reach the opposite poles and begin to unravel. Nuclear envelopes form around the chromosomes.
Cytokinesis is the second part of the mitotic phase, and this is when the cell is physically separated (divided) into two daughter cells.
Meiosis is the fertilization of the daughter cells from mitosis. This produces reproductive cells when the nucleus divides into four nuclei, and chromosomes exchange genetic material (known as “crossing over”).
While mitosis produces two daughter cells that are genetically identical, meiosis creates four cells that are genetically unidentical. Meiosis produces gametes (sperm and egg cells) to form a zygote (a diploid cell with the full number of chromosomes).
Basic Biochemistry of Life
This section tests your knowledge on basic biological functions and building blocks for life.
Let’s talk about a concept that may come up on the test.
Cellular Respiration
Cellular respiration is the process of breaking down sugar so that the cell can use it for energy. Cells of animals, plants, fungi, and protists use oxygen to break down food molecules to create ATP, the chemical compound that cells use for energy.
Cell respiration begins with Step 1. Glucose, the basic energy source for the cell. Then, the cell begins Step 2: Glycolysis (the breakdown of glucose).
Step 3: The Link Reaction, which takes place inside the mitochondria. Link reaction allows the cell to start Step 4: the Krebs Cycle, which converts ADP to ATP by removing oxygen and producing carbon dioxide and water.
Step 5: Most of the ATP is made during the electron transport chain (ETC).
The basic formula for this is:
Glucose (sugar) + oxygen –> carbon dioxide + water + energy (as ATP)
This process is essential for life so that the cell has the energy to function. Cells can’t process just glucose alone but need to convert it to ATP to use it for energy.
Basic Genetics
This section tests your knowledge on the basic principles of genetics.
Let’s discuss a concept likely to show up on the test.
Mendelian Inheritance
Gregor Mendel is known as the “father of genetics.” He discovered the 3 essential laws of inheritance:
- Segregation: Every organism contains two alleles for each trait, one from each parent. An allele is a pair of genes on a chromosome that determine a characteristic. For example, an allele can determine eye color.
- Independent assortment: Alleles for separate traits are inherited independently of one another. The inheritance of one trait is not dependent on the inheritance of another.
- Dominance: Genes are either dominant or recessive. The law of dominance states that an organism with alternate forms of a gene (one dominant and one recessive) will always express the form that is dominant.
The hereditary information of a gene is known as its genotype. For example, the genotypes for brown eyes and blue eyes are different codes based on dominant and recessive alleles. The phenotype is the physical (outward) expressed trait of those characteristics. We see brown or blue eyes, and that is the physical expression of the gene. Whether a person has a Widow’s peak is also a phenotype.
Theory and Key Mechanisms of Evolution
This section tests your knowledge on taxonomy and evolution, including how scientists chart organism families, nomenclature, and chart evidence for evolution.
Hierarchical Classification Scheme

This section tests your knowledge of the classification of organisms (taxonomy).
Here’s a concept that is likely to be on the test.
Classification Characteristics
Scientists classify organisms into one of five families or groups: bacteria, animals, plants, fungi, and protists. The classifications are based on the organism’s cellular structure.
Bacteria: Bacteria are prokaryotes, which means that all the DNA is found in the nucleoid and is not surrounded by a cell membrane. Bacteria have no cell nucleus.
Animals: Animal cells do not have a cell wall. Instead, they are encased in a thin cell membrane. Animal cells do not have chloroplasts and cannot undergo photosynthesis.
Plants: All plant cells have a hard cell wall made mostly of cellulose. This helps give plants their structure. Plant cells also have chloroplasts which are used in photosynthesis.
Fungi: Fungi cells have a cell wall, similar to plants. However, they are different from plants because their cell walls are made of chitin, a compound found in the shells of crustaceans. Fungi do not have chloroplasts and do not undergo photosynthesis.
Protists: Protists are unicellular organisms. They are eukaryotes, but they are single-celled. Unlike plant or animal cells, protists can move on their own, usually by using small tails (flagella), tiny hairs (cilia), or arm-like extensions of the cell (pseudocilia). A protist is a complete, single-celled organism.
Major Structures of Plants and Their Functions
This section tests your knowledge on plant biology, structure, and growth.
Basic Anatomy and Physiology of Animals, including the Human Body
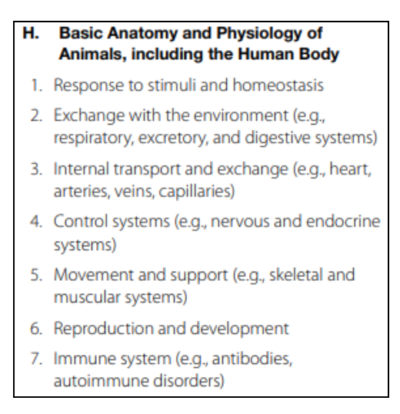
This section tests your knowledge on anatomy and physiology of animals.
Let’s discuss a concept that will more than likely appear on the test.
Homeostasis
Homeostasis is the process toward a stable equilibrium between elements, especially in physiology. Think of it as maintaining a constant internal environment. In humans, an example of homeostasis is maintaining a constant body temperature by releasing heat or maintaining healthy blood pressure.
Homeostasis controls important functions at the cellular level, and all the way through entire organs and organ systems.
When homeostasis is not achieved, it is imbalanced. Homeostatic imbalance results in disease or toxicity. An example of homeostatic imbalance is the disease of diabetes, where the endocrine system has difficulty maintaining the appropriate blood glucose levels.
This is some information about the Life Science portion of the Praxis®️ General Science exam.
Praxis®️ General Science: Earth and Space Science
Overview
The Earth and Space Science content category has about 27 multiple-choice questions, which account for about 20% of the Praxis®️ General Science test.
This part has five sections:
- Physical Geology
- Historical Geology
- Earth’s Bodies of Water
- Meteorology and Climate
- Astronomy
So, let’s start with Physical Geology.
Physical Geology

This section tests your knowledge on geology and its impact on the physical world.
Let’s go through a concept very likely to appear on the test.
Plate Tectonics
Plate tectonics is the theory that describes how the earth is composed of seven large plates, and how their movement across the earth’s mantle shapes the geological features we see today. We have gathered evidence from fossils, glaciers, and coastlines to reveal how the plates once fit together.
- Folding: Refers to the changes in shape in rocks when they fracture or crumble into “folds.” Think of a fold as a bend in the rock caused by huge compressing forces. These folds occur at Earth’s crust.
- Faulting: When plates move and shift, this can create large spaces between the tectonic plates. These spaces are known as faults.
- Continental drift: Over millions of years, the continental plates have drifted across the mantle. Continents have formed into new configurations due to drift.
- Seafloor spreading: Seafloor spreading also helps explain continental drift. As the oceanic plates separate, magma rises up from the earth’s core, which cools and creates new seabed. This changes the landscape of our ocean floor and also causes further continental drift.
- Magnetic reversals: Plate tectonics control the changes in Earth’s magnetic field. The magnetic reversal means Earth reverses its polarity (north becomes south, south becomes north). This happens at Earth’s liquid core and becomes apparent through seafloor spreading (when new magma emerges and then cools).
Historical Geology

This section tests your knowledge on geologic principles and how we use fossil evidence to prove it.
Here is a concept that is likely to pop up on the test.
Uniformitarianism
Uniformitarianism is remembered with this phrase, “the present is the key to the past.” This states that our current landscape developed as a result of slow geologic and geomorphic processes.
Earth’s Bodies of Water

This section tests your knowledge on bodies of water and how they shape our landscape.
Let’s discuss a concept that is likely to be on the test.
Effects on Earth’s Systems
Water is a powerful agent in shaping the Earth we know today. The amount of atmospheric water vapor controls rainfall, sea levels, and sea surface. These, in turn, shape our weather and climate through the water cycle and Earth’s heat cycles.
Density Changes on Freezing: Water expands when it freezes, making it less dense than water. This means that frozen water in the form of ice caps, icebergs, or ice shelves will float on water. When water is a liquid, the molecules are packed relatively close to one another. When it freezes, the molecules form together in a way that they are farther apart than in liquid form.
High Heat Capacity: Specific heat refers to the amount of heat (in calories) needed to raise the temperature of 1 gram of water by 1 degree Celsius.
Water has a high heat capacity—in fact, it has the highest heat capacity of any liquid. This is caused by the hydrogen bonding in the molecules. This means that water can absorb a lot of heat without a significant rise in temperature. When we consider that water covers about 70% of Earth’s surface, with a high specific heat, it takes both a long time to heat and a long time to cool.
Polar Solvent: This is a liquid that has molecules with a slight electrical charge because of its shape (positive and negative charges). Water is a polar solvent.
Hydrogen Bonding: The reason water has such a high specific heat, and the reason why ice is less dense than water, is due to hydrogen bonding. Polar molecules (water) have a weak, partially negative charge at one end of the molecule. The force of attraction creates a strong covalent hydrogen bond.
Meteorology and Climate

This section tests your knowledge on meteorology and climatology.
Let’s discuss a concept that will more than likely appear on the test.
Earth’s Atmosphere
The atmosphere refers to the layer of gases that surround the Earth, which is held in place by gravity. Earth’s atmosphere is made of nitrogen (78.1%), oxygen (20.9%), argon (0.9%), water vapor, and trace amounts of other gases.
There are five main layers of the atmosphere:
- Troposphere: This is the lowest part. It’s where we live. Troposphere begins at ground level and reaches about 10 km (6.2 miles, or 33,000 feet) up. Most clouds appear here, and about 99% of our water vapor is in the troposphere.
- Stratosphere: This is the next layer up. This extends from the end of the troposphere (10km) up to about 50 km above the ground. The ozone layer is found here. Commercial planes fly in the lower part of the stratosphere. This layer actually gets warmer the higher up you go!
- Mesosphere: The mesosphere is above the stratosphere. This extends to about 85 km high. Temperatures here begin to get colder. Here, air is too thin to breathe and air pressure drops.
- Thermosphere: This is at about 90 km high. This is a very thin layer that absorbs the sun’s high-energy x-rays and UV rays. Air here is so thin that it would feel freezing to us, even though the temperature is quite hot. Many satellites orbit our earth in the thermosphere, and it is the beginning of what we would call “outer space.”
- Exosphere: This is the final layer of earth’s gaseous protective layers. The air here is extremely thin, making this the most space-like of the layers. There is no clear boundary between where the exosphere ends and outer space begins. The exosphere begins somewhere between 100,000km (62,000 miles) and 190,000km (120,000 miles) up.
Astronomy

This section tests your knowledge on the solar system, the earth-moon system, and major features of the universe.
This is a concept that is likely to be on the test.
Asteroids, Meteoroids, Comets, and Minor Planets
Asteroids are small objects that orbit the sun. These can be as small as a few feet wide to several miles.
Meteoroids are small rocky objects in outer space, much smaller than an asteroid. These range in size from small grains to about one meter wide.
Comets are icy balls of rock that grow a tail as they travel close to the sun. As comets travel, they heat up, causing gas and dust to fall from the body and create the iconic “tail.”
Minor planets are objects in space that orbit around the sun. They aren’t quite planets and they aren’t quite comets, so they are classified as “minor planets.” Minor planets can be dwarf planets or asteroids.
And that’s some basic info about the Earth and Space Science content category of the Praxis®️ General Science exam.
Praxis®️ General Science: Science, Technology, and Society
Overview
The Science, Technology, and Society content category has about 15 multiple-choice questions, which account for about 11% of the test.
This part has four sections:
- Impact of Science and Technology on the Environment and Society
- Major Issues associated with Energy Production and the Management of Natural Resources
- Application of Science and Technology in Daily Life
- Impact of Science on Public Health Issues
So, let’s start with the Impact of Science and Technology on the Environment and Society.
Impact of Science and Technology on the Environment and Society

This section tests your knowledge on environmental and physical sciences.
Let’s discuss a concept that will more than likely appear on the test.
Loss of Biodiversity
Biodiversity refers to the variety of plant and animal life in our ecosystem. Loss of habitat, pollution, overpopulation, deforestation (a type of habitat loss), and global warming all contribute to loss of biodiversity. These agents stress habitats, causing organisms to have to compete for resources, which can lead to resource depletion or unsustainable population growth/decline.
Major Issues associated with Energy Production and the Management of Natural Resources

This section tests your knowledge on issues associated with energy and natural resource management.
Let’s talk about a concept that has a strong chance of being on the test.
Power Generation
There are nine major ways we get our energy. These energy types are either renewable or non-renewable.
Renewable energy sources are things that can be replenished naturally in a short period of time. There is no shortage of these energy sources, or they can be renewed quickly. Examples include solar, wind, water, biomass, and geothermal energies. There is no set number of these energy sources (there is no finite number of wind gusts or sun rays!). We can continue to harvest these energies because they are quick to renew. Think of renewable energy sources like this: there is a set amount today, but there will be more available tomorrow. The upside to these resources is that they can be generated quickly. However, it can be expensive to set up initial ways to harvest these energy sources.
Non-renewable energy sources are things like nuclear power, coal, oil, and natural gas. These are available, but only in limited amounts. There is a finite amount of coal and oil on earth, and these can’t be renewed quickly. For example, fossil fuels (coal, oil, and gas) were created from dead plant and animal matter from millions of years ago. It takes millions of years for fossil fuels to form, so this is not something that can be replenished quickly. While these sources are lucrative and cheap to use, the major downside is that they are not renewable, so they will not be sustainable for long-term usage. Nuclear energy is harvested from Uranium from the Earth’s crust. Like fossil fuels, there is only a set amount of it available, so it is non-renewable. Think of non-renewable energy like this: whatever amount is on the earth now is all we have. Renewable energy is constantly being added to our environment, so we can continue to use these without worrying that we will use it all up.
Application of Science and Technology in Daily Life

This section tests your knowledge on science applications in everyday life, including technology, communication, crime, and consumer products.
This is a concept that will more than likely appear on the test.
Nanotechnology
Nanotechnology is something we live with every day, and probably don’t even think about. Nanotechnology is a part of science that deals with things at the atomic and molecular levels. This means that things are about 100 nanometers across. “Nano” means “billionth,” so 1 nanometer = 1 billionth of 1 meter. It is much smaller than the microscopic scale. Medicine (medications that target cancer cells), electronic sensors, the display on your computer screen, cleaning polluted water, and even sunscreen are all examples of how we use nanotechnology today.
Impact of Science on Public Health Issues

This section tests your knowledge on the science behind public health and medical technology.
Let’s discuss a concept that will more than likely appear on the test.
Vaccines
A vaccine is used as a treatment to make the body stronger against a particular disease or infection. We administer vaccines to help prepare your immune system to fight the full disease. A vaccine is a small amount of weakened viruses (like chickenpox) given to a patient to help stimulate an immune response. This allows your body to develop the ability to fight against the disease without contracting the full sickness.
The use of vaccines revolutionized public health. By giving a patient an inactive or weakened strain of a disease, the body is able to naturally create the defenses to fight it off. This has allowed for improved public health at very large scales. Thanks to vaccines, we have eliminated major public health threats, such as polio, measles, mumps, and rubella.
And that’s some basic info about the Science, Technology, and Society content category of the Praxis®️ General Science exam.