TExES Chemistry 7-12 Competencies
Domain I of the TExES Chemistry 7-12 test includes about 24 multiple-choice questions. There are four competencies within this domain.
Let’s explore some specific topics from within this domain.
Staying Safe in Labs
Lab safety is the primary aim of any chemistry experiment. The following list reviews common safety practices.
- Avoid cross-contamination of chemicals by disposing of excess rather than returning chemicals to their original containers.
- Dispose of chemicals in the appropriately labeled containers; for example, “aqueous liquid waste,” “glass waste,” “biohazard waste,” “sharps waste.”
- Wear personal protective equipment, including goggles, lab coat, gloves, and closed-toe shoes at all times in the lab. Take extra care when working with glass, corrosive chemicals, and open flames.
- To maintain good hygiene and prevent cross-contamination, always wash hands before experiments and after removing gloves.
- Never open a chemical container in the direction of someone’s face. When possible, direct open containers toward the back of a fume hood.
- Never use chipped glassware, and exercise caution when cleaning up broken glass.
- Never eat, drink, or store consumables in the lab.
- Never breathe in chemicals. To waft chemical odors, gently wave the air near the chemical toward the face.
- Never taste chemicals or put chemicals in your mouth, nose, or eyes.
- Keep electrical outlets uncluttered, and prevent them from getting wet.
- In the case of a chemical spill, accident, or emergency, notify the teacher or get help immediately.
Scientific Inquiry
Scientific inquiry refers to methods of learning about the world that are based on observations, experimentation, and evidence-based conclusions. Scientific inquiry is methodological, systematic, reproducible, and often (but not always) quantitative. Both deductive and inductive reasoning can be used in scientific inquiry.
In contrast, nonscientific inquiry methods of learning make conclusions based on personal experience, authority figures, and preconceived biases.
Independent vs. Dependent Variables
Scientific inquiry involves testing the effects of the independent variable on the dependent variable.
The independent variable
is the thing being changed to affect the dependent variable. The dependent variable
, sometimes called the response variable, is the thing that is being measured after being affected by the independent variable. The independent variable and dependent variable are usually named in the title of an experiment; for example, “How does light intensity (independent variable) affect the rate of photosynthesis (dependent variable)?”
Scientific Notation
Scientific notation is a shorthand method of expressing numerical values that contain lots of zeros. Numbers that are much greater than 1 can be shortened by dividing out factors of 10 until the base number is between 1 and 10. Likewise, numbers that are much smaller than 1 can be shortened by multiplying factors of 10 until the base number is between 1 and 10.
Example 1:
Express 24000000000000 in scientific notation.
Move the decimal point to reach a base number between 1-10, in this case, 2.4. Then multiply by 10 to the power of 13 and add one power of 10 for each decimal place.
= 2.4 x 10 13 Example 2:
Express 0.000000000000000000000456 in scientific notation.
Move the decimal point to reach a base number between 1-10, in this case, 4.56. Then multiply by 10 to the power of -22 and subtract one power of 10 for each decimal place.
= 4.56 x 10 -22 Systems Model
A system
is a set of components that work together in a complex network. Examples of systems in chemistry include biochemical mechanisms such as the hormone signaling pathway, chemical reactions in closed containers, and nitrogen cycling through the environment.
Systems models
are a method for organizing and visualizing individual parts of a larger, more complex system.
Components of a system model include:
- Boundaries – definitions of where the system begins and ends
- Inputs – data and parameters that go into constructing the model
- Outputs – decisions and consequences that are produced by the model
- Feedback loops – parameters in which the outcome goes back into the system
- Subsystems – related subsystems contained within the main system of study
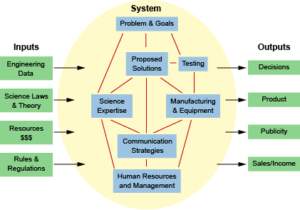
The following example applies the systems model to designing a long-term environmental monitoring study for an estuary region.
- Boundaries
- Geographical region of the study
- Time period for the study
- Inputs
- Parameters being measured (pH, salinity, turbidity, biodiversity)
- Sampling sites within the estuary
- Availability of financial resources and research assistants
- Roles for stakeholders in local government/industry
- Outputs
- Reports on ecosystem health for this estuary
- Scientific publications
- Experience for research assistants/students
- Changes to policy or industrial practices
- Feedback loops
- Ongoing ecosystem health checks to evaluate the effects of policy changes
- Subsystems
- Aquaculture practices within the estuary
- Environmental chemistry in the water
- Nearby real estate development or agricultural activities
TExES Chemistry 7-12 Domain II: Matter and Energy
Domain II includes about 41 multiple-choice questions. There are seven competencies within this domain.
Let’s explore some specific topics from within this domain.
Gas Laws
Gas laws describe relationships between molecules in the gas phase. Gas phase particles can be thought of as individual particles moving randomly in an enclosed container. There are four main laws that govern the behavior of gases.
Charles’s law
describes the relationship between volume (V) and temperature (T) for a gas at constant pressure (P).
Volume and temperature are directly related.
As V ↑, then T↑; as V↓, then T↓
If a system experiences a change in volume or temperature, a proportional relationship between the initial and final conditions is given by
V1/T1 = V2/T2 Boyle’s law
describes the relationship between volume (V) and pressure (P) for a gas at a constant temperature (T). Volume and pressure are inversely related.
As V ↑, then P↓; as V,↓ then TP↑
If a system experiences a change in volume or pressure, a proportional relationship
between the initial and final conditions is given by
P1V1 = P2V2 Avogadro’s law
describes the relationship between volume (V) and moles of gas (n) for a gas at constant temperature (T) and pressure (P). Volume and moles of gas are directly related.
As V ↑, then n↑; as V↓, then n↓
If a system experiences a change in volume or moles of gas, a proportional relationship between the initial and final conditions is given by
V1/n1 = V2/n2The combined gas law incorporates all of the relationships in the previous three laws to describe the relationship between volume (V), pressure (P), and temperature (T) for a given gas sample (constant moles).
If a system experiences a change in V, P, or T parameters, a proportional relationship between the initial and final conditions is given by
P1V1/T1 = P2V2/T2
Endothermic vs. Exothermic
Chemical reactions transfer heat as molecular bonds break and form between atoms. The amount of heat that is transferred depends on the strength of the bond.
Exothermic reactions
are chemical reactions that
release heat,
which means that the reactants are higher energy than the products. When the products form, the extra energy is transferred to the environment as heat energy. The reaction coordinate diagram shows the general progression of an exothermic reaction.

Endothermic reactions
are chemical reactions that
absorb heat, which means that the reactants are lower energy than the products. When the products form, the extra energy is transferred from the environment as heat energy. The reaction coordinate diagram shows the general progression of an endothermic reaction.

History of the Periodic Table
The periodic table is foundational to all chemical science. It organizes the elements, pure atomic substances, in a way that creates periodic trends and groups with similar properties.
Discovery of Elements
The discovery of phosphorus by Robert Boyle in 1680 is believed to be the first published finding of a pure element. Over a century later in 1789, Antoine Lavoisier defined an element as a substance that does not break down to a simpler substance in a chemical reaction. Lavoisier named oxygen, nitrogen, hydrogen, phosphorus, mercury, zinc, and sulfur and classified metal and nonmetal elements.
Classification and Organization
Dimitri Mendeleev, a Russian chemist, is widely acknowledged as the father of the modern periodic table. In 1869 Mendeleev arranged the known 63 elements by atomic weight. This new organization left spaces for undiscovered elements to be added in the future. In the late 1890s, the discovery of new inert gases by William Ramsay and Lord Rayleigh prompted the addition of an additional noble gases group (He, Ne, Ar, Kr, Xe, Rn) to the periodic table.
Rare Earth Elements
In 1905 a Swiss chemist named Alfred Werner solved another part of the periodic puzzle. Rare earth elements (lanthanides and actinides) were difficult to place in the original table because their number and size were still largely unknown. Werner expanded the periodic table by adding new columns for the lanthanide rare-earth elements. Actinide rare-earth metals were added several decades later after experiments by chemist Glenn Seaborg and physicist Edwin McMillan, both Americans.
Recent Additions
The most recent additions to the periodic table include four new elements added in 2015 (nihonium-113, moscovium-115, tennessine-117, and oganesson-118). New elements are created by fusing atomic nuclei to form superheavy atoms. These artificial elements are very unstable and may only exist for a fraction of a second before they undergo radioactive decay.
Types of Bonds
Intramolecular bonds are chemical bonds that connect atoms within a molecule. Intramolecular bonds are stronger than the intermolecular bonds between molecules.
There are three main types of intramolecular bonds:
- Covalent bond– valence electrons between adjacent atoms areshared
- Single covalent bond – one pair of electrons are shared, as in methane (CH 4)
- Double covalent bond – two pairs of electrons are shared, as in diatomic oxygen (O 2)
- Triple covalent bond – three pairs of electrons are shared, as in diatomic nitrogen (N 2)
- Ionic bond– valence electrons between adjacent atoms aretransferred
- Creates a strong electrostatic attraction between the positively charged cation (electron donor) and the negatively charged anion (electron acceptor)
- Examples include salts like sodium chloride (NaCl), potassium iodide (KI), and magnesium oxide (MgO)
- Metallic bond– valence electrons aredelocalizedthroughout the metal
- Electrostatic forces between the positively charged metal nuclei and the negatively charged electrons hold adjacent metal nuclei in place
- Conduction of electric current from a flow of charged electrons through the metal
- Examples of metallic bonding include pieces of solid metal like Silver (Ag), Copper (Cu), and Sodium (Na).
Kinetic Molecular Theory – Arrhenius Equation
Kinetic molecular theory explains how the behavior of particles in the gas phase are related to the observable quantities of pressure, volume, and temperature. Kinetic molecular theory makes a number of assumptions about gas molecules.
Assumptions of Kinetic Molecular Theory
- Gas particles are in constant, random motion, and they move in straight lines until they collide with other particles or the sides of the container.
- Gas particles are inert and do not experience any attractive or repellent forces.
- Collisions between gas particles are completely elastic.
- The size of the gas particles is negligible compared to the volume of the container.
- The average kinetic energy of the gas particles depends solely on temperature. Therefore there is no molecular motion in a system at a temperature of 0 K.
The Arrhenius equation uses the assumptions of kinetic molecular theory to calculate the
reaction rate constants for chemical reactions.
The Arrhenius equation is expressed by
k = Ae -Ea/RT where
k ⇒ reaction rate constant
A ⇒ pre-exponential factor
E a⇒ activation energy (J/ mol)
R ⇒ universal gas constant; 8.314 J/mol K
T ⇒ temperature (K)
Since the pre-exponential factor is a constant and RT is kinetic energy, the reaction rate is inversely proportional to the ratio of activation energy to kinetic energy.
Example:
Near room temperature (293 K), reaction rate doubles with approximately a 10-degree rise in temperature. Use the Arrhenius equation as a guide to show that raising the temperature from 290 to 300 can cause doubling for a chemical reaction with an activation energy of 65000 j/mol.
- Start with the Arrhenius equation:
k = Ae-Ea/RT
- Summarize the equation to focus on the dependent terms:
k ~ e-Ea/RT
- Substitute the relevant values for each temperature and compare the results:
kT=290 ~ e-Ea/RT
= e-65000/(8.31 x 290) = 1.932 x 10-12 = k @ 290 K
kT = 300 ~ e-Ea/RT = e-65000/(8.31 x 300) = 4.749 x 10-12 = k @ 300 K
The rate at the higher temperature is about 2.4 times the rate at the lower temperature.
Nomenclature – Acids
Naming conventions for acids depend on the number of atoms and the identity of the conjugate base.
- If the acid is diatomic, use the prefix “hydro-” and the suffix “-ic acid.”

Examples:

- If the acid is polyatomic AND the conjugate base anion ends in “-ate,” use the base name and the suffix “-ic acid.”

Examples:
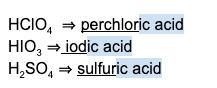
- If the acid is polyatomic AND the conjugate base anion ends in “-ite,” use the base name and the suffix “-ous acid.

Examples:

Molarity
Molarity is a measure of concentration expressed by units of moles of solute per liter of solution (mol/L or M). Molar concentration conveys the number of solute molecules in the solution. Moles can be converted to grams using molar mass.
Example:
Find the molarity, rounded to the nearest integer, of a solution that contains 361 g glucose (C 6H 12O 6) dissolved in 200 mL of water.
- Calculate the moles of glucose in 1.4 g glucose:
molar mass C = 12.010 g/mol
molar mass H = 1.007 g/mol
molar mass O = 15.999 g/mol
molar mass glucose = 6(12.010 g/mol) + 12(1.007 g/mol) + 6(15.999 g/mol)
= 180.128 g/mol
? mol glucose = 361 g glucose (1 mol glucose/180.128 g)
= 2.004 mol glucose
2. Divide by solvent volume in liters:
200 mL = 0.2 L
2.004 mol glucose/0.2 L water = 10 M
The solution is about 10 M glucose in water.
Enthalpy
Enthalpy is a thermodynamic principle that describes the total capacity of a system to release heat. The SI unit for enthalpy is joule per kilogram (J/Kg), which is the energy released as heat per kilogram of substance. In equations, such as the Gibb’s free energy equation, enthalpy is denoted by H and a change in enthalpy is denoted by ΔH.
ΔG = ΔH – TΔS
The Gibbs free energy equation relates the change in enthalpy to the total free energy change of a system. In addition to enthalpy, the total change in free energy also depends on the temperature (T) multiplied by the change in entropy (ΔS), another thermodynamic principle.
If a system undergoes a positive change in enthalpy (ΔH > 0), then the system has gained heat energy from the environment.
If a system undergoes a negative change in enthalpy (ΔH < 0), then the system has lost heat energy to the environment.
TExES Chemistry 7-12 Domain III: Chemical Reactions
Domain III has about 23 multiple-choice questions. There are four competencies within this domain.
Let’s explore some specific topics from within this domain.
Chemical Equilibrium
Without additional external forces, chemical reactions will tend toward a state of equilibrium. A reaction has reached chemical equilibrium
when the products and reactants form at an equal rate and have a net concentration change of zero. Chemical equilibrium is a form of dynamic equilibrium. In dynamic equilibrium
the system is still in motion, but because the forward rate is equal to the backward rate, there is no
net
change over time.
A reaction at equilibrium proceeds in the forward and backward direction at the same rate, which is denoted by an antiparallel arrow.
A + B ⇆ C + D
In the preceding equation, the rate of formation for products C and D from A and B is equal to the rate of formation for reactants A and B from products C and D.
Even though the rate of the forward and backward reactions are equal, the concentrations of the chemicals can differ at equilibrium. The equilibrium constant (K) is the ratio of the concentration of products to reactants, with each species raised to the power of its coefficient.
For the equation
2A + 1B ⇆ 3C + 2D
K = [C] 3 [D] 2 /[A] 2 [B]
If K > 1, then products dominate the reaction at equilibrium.
If K < 1, then reactants dominate the reaction at equilibrium.
If K = 1, then there are the same amount of products and reactants at equilibrium.
Acids, Bases, and Salts
Salts
are ionic compounds, which means they are composed of a positively charged cation and a negatively charged anion. Solid salts have a crystalline structure, but they can have a variety of colors, odors, and tastes depending on the ions involved. For example, sodium chloride (NaCl) is used as table salt and has a salty flavor. However, lead (II) acetate (Pb(CH 3COO) 2), a toxic lead compound, was used by ancient Romans as a sweetener.
Salts are classified according to how they affect pH when dissolved in water.
Neutral salts
are formed by spectator ions in a neutralization reaction between a strong acid and a strong base. Dissolving a neutral salt in an aqueous solution does not affect the pH. A neutral salt in pure water should have a pH equal to 7.
NaOH (strong base) + HCl (strong acid) → H 2O (liquid water) + NaCl (neutral salt)
Examples of neutral salts:
sodium chloride (NaCl)
potassium nitrate (KNO 3)
calcium iodide (CaI 2)
Acidic salts
donate H + ions, resulting in a solution with a pH less than 7.
Examples of acidic salts:
ammonium iodide (NH 4I)
sodium dihydrogen phosphate (NaH 2PO 4)
potassium hydrogen sulfate (KHSO 4)
Basic salts
accept H + ions, resulting in a solution with a pH greater than 7.
Examples of basic salts:
potassium carbonate (K 2CO 3)
sodium sulfide (Na 2S)
sodium acetate (NaCH 3COO)
Nuclear Energy: Fission vs. Fusion
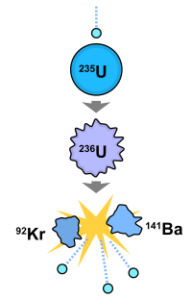
Nuclear fission
splits heavy atomic nuclei into smaller, lighter nuclei. Nuclear fission releases neutrons, heat energy, and gamma radiation.
In the diagram below, uranium-235 gains an electron, causing it to become uranium-236. The heavier uranium nucleus is less stable and higher energy, which drives the fission reaction. Uranium-236 splits into krypton-92 and barium-141, releasing extra neutrons, kinetic energy, and high- energy gamma radiation.
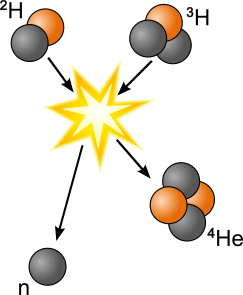
Nuclear fusion
combines small atomic nuclei into one heavy atomic nucleus. Nuclear fusion that produces a light nucleus is exothermic, and nuclear fusion that produces a heavy nucleus is endothermic.
In the diagram below, deuterium (1 proton + 1 neutron) is fused with tritium (1 proton + 2 neutrons) to produce helium (2 protons + 2 neutrons). An extra neutron and kinetic energy are released by the reaction.
Balancing Chemical Reactions
The law of conservation of mass
states that mass cannot be created or destroyed, and the mass of a closed system will stay constant. In chemical reactions bonds break and form between different atoms but the atoms themselves do not change.
A balanced chemical equation follows the law of conservation of mass. Equations are balanced by adding
coefficients
to signify how many molecules of each compound are used in the reaction.
Example 1:
Balance the following chemical equation for the decomposition of hydrogen chloride:
HCl (g) → H 2(g) + Cl 2(g)
Write out the number of atoms from each side of the equation.
The reactants have 1 atom each of H and Cl and the products have 2 atoms each of H and Cl.
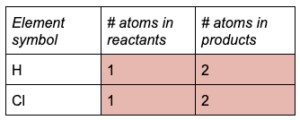
Find the coefficient needed to balance each atom.
To balance the reactants and the products, the reactants need to be multiplied by 2.
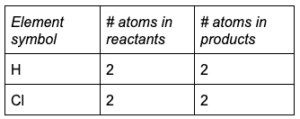
Rewrite the balanced chemical equation, and check that the coefficients obey the law of conservation of mass:
2
HCl (g) → H 2(g) + Cl 2(g)
Example 2:
Balance the following chemical equation for the formation of water vapor from oxygen and hydrogen:
O 2(g) + H 2(g) → H 2O
(g)
Write out the number of atoms from each side of the equation.
The reactants have 2 atoms each of O and H, and the products have 2 atoms of H and one atom of O.
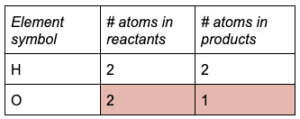
Find the coefficient needed to balance each atom.
From the table, O in the products needs a coefficient of 2.
Rewrite the chemical equation and check that the coefficients obey the law of conservation of mass:
O 2(g) + H 2(g) →
2
H 2O
(g)
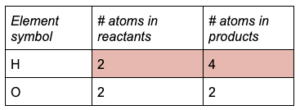
The coefficient also doubled the amount of H in the products, so the equation is still
unbalanced. Add another coefficient of 2 to H in the reactants.
Rewrite the balanced chemical equation and recheck that the coefficients obey the law of conservation of mass:
O 2(g) +
2
H 2(g) →
2
H 2O
(g)
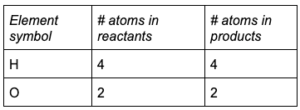
Example 3:
The following equation shows how plants make glucose from carbon dioxide, water, and sunlight. Balance the compounds in this equation:
CO 2(g) + H 2O (g) + (energy) → C 6H 12O 6(s) + O 2(g)
Write out the number of atoms from each side of the equation.
Energy is not an atom with mass so it is not included in balancing equations.
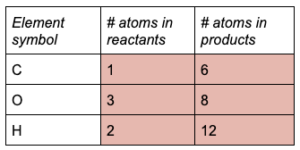
Find the coefficient needed to balance each atom.
Start with C and H because they are in one compound from the products and reactants. Give CO 2and H 2O a coefficient of 6.
Rewrite the chemical equation and check that the coefficients obey the law of conservation of mass.
Notice that the coefficients in the reactants changed the number of atoms of oxygen:
6
CO 2(g) +
6
H 2O (g) + (energy) → C 6H 12O 6(s) + O 2(g)
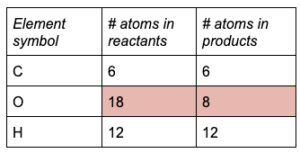
Find the coefficient needed to balance oxygen.
Adding a coefficient of 6 to O 2in the products will give 18 atoms of O on each side.
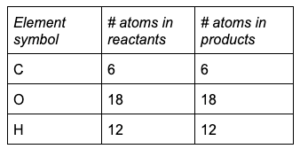
Rewrite the balanced chemical equation and recheck that the coefficients obey the law of conservation of mass:
6
CO 2(g) +
6
H 2O (g) + (energy) → C 6H 12O 6(s) +
6
O 2(g)
Balancing Chemical Reactions – Redox
Reduction oxidation reactions are chemical reactions that involve the transfer of electrons between different compounds.
Compounds that
accept electrons
are
reduced
, which
decreases the oxidation number
.
Compounds that
donate electrons
are
oxidized
, which
increases the oxidation number
.
Example 1:
Add electrons to balance the reduction half-reaction for silver:
Ag 2+ → Ag (s)
Add electrons to the more positive side of the equation to reduce Ag 2+ to Ag:
Ag 2+ + 2e – → Ag (s)
Example 2:
Balance the following redox reaction between iron and platinum:
Fe 3+ (aq) + Pt (s) → Pt 2+ (aq) + Fe (s)
Separate the half-reactions and add electrons to the more positive side.
Reduction:
Fe 3+ (aq) +
3e
–
→ Fe (s)
Oxidation:
Pt (s) → Pt 2+ (aq) +
2e
–
Multiply both equations by a coefficient to achieve the smallest common multiple for the electrons.
Reduction:
(Fe 3+ (aq) + 3e – → Fe (s) )
x 2
= 2Fe 3+ (aq) + 6e – → 2Fe (s)
Oxidation:
(Pt (s) → Pt 2+ (aq) + 2e – )
x 3
=3Pt (s) → 3Pt 2+ (aq) + 6e – Combine redox half reactions and divide out electrons:
2Fe 3+ (aq) + 3Pt (s) → 2Fe (s) + 3Pt 2+ (aq)
Example 3:
Balance the following redox reaction in acidic conditions:
Fe 2O 3(s) + Br – (aq) → Br 2(l) + Fe 2+ (aq)
Separate the half-reactions and balance atoms EXCEPT H and O.
Reduction:
Fe 2O 3(s) →
2
Fe 2+ (aq)
Oxidation:
2
Br – (aq) → Br 2(l)
First, add H 2O to balance O and then add H + to balance H. Check that each half-reaction obeys the law of conservation of mass.
Reduction:
6H + (aq) + Fe 2O 3(s) → 2Fe 2+ (aq) +
3H
2
O (l)
Oxidation:
2
Br – (aq) → Br 2(l)
Add electrons to the more positive side to balance the total charge (including H + )
Reduction:
reactant charge = +6
product charge = +4
2e
–
+ 6H + (aq) + Fe 2O 3(s) → 2Fe 2+ (aq) + 3H 2O (l)
Oxidation:
reactant charge = -2
product charge = 0
2Br – (aq) → Br 2(l) +
2e – Combine half-reactions and divide out electrons and check that mass and charge are balanced.
2Br – (aq) + 6H + (aq) + Fe 2O 3(s) → 2Fe 2+ (aq) + 3H 2O (l) + Br 2(l)
TExES Chemistry 7-12 Domain IV: Science Learning, Instruction and Assessment
Domain IV has about 12 multiple-choice questions. There are two competencies within this domain.
Let’s explore some specific topics from within this domain.
Assessment Validity
Assessments aim to evaluate student understanding both on an ongoing basis and at key points in the curriculum (e,g., units, quarters, semesters). Strong assessments share the following characteristics.
Congruency
Congruent assessment refers to the relationship between the instructional material, the learning objectives, and the assessment methods. Mismatches between instruction, curriculum, and assessments can lead to inaccurate results and gaps in student knowledge. For an effective congruence model, teaching should encompass the largest breadth of material, students will learn a portion of the material taught, and assessments will test a portion of the material learned.
Reliability
Assessment reliability describes the consistency of assessments. Student scores for the same assessment should be consistent and depend only on the learned material. To best measure student achievement, scores should depend only on student knowledge of the assessment content.
There are several ways to measure the relative reliability of assessments.
- Test-retest – measures the relative reliability of the same assessment given at two different times to the same group of students
- For example, a seventh grade class that takes the same science assessment one week apart should score in a consistent range.
- Alternate form – measures the relative reliability of two forms of the same assessment
- For example, If half a class takes test form A and half the class takes test form B, scores from both forms should fall into a consistent range.
- Internal consistency – measures the relative reliability of questions within one assessment
- For example, students should miss questions at a consistent rate throughout the whole assessment.
Validity
Assessment validity describes how well an assessment tests the intended material. Valid assessments have questions that are targeted to specific points within the subject material. Confusing wording, vague distractors, and test anxiety can all affect assessment validity.
There are several ways to measure the relative validity of assessments.
- Content – measures the correlation between the assessment content and curriculum objectives
- For example, a unit assessment for acid/base chemistry may include questions on pH calculations, titrations, and buffering, but it should not include questions on periodic trends.
- Criterion – measures the correlation between assessment scores and a non-assessment progress indicator
- For example, scores from an assessment on experimental practice should be in agreement with student performance in an experimental lab activity.
- Construct – measures the correlation between assessment performance and predictions by the instructor
-
- For example, if the instructional material does not include information on photosynthesis, then students should score poorly on photosynthesis assessment questions.
Absence of Bias
The absence of bias in assessment means that diversity characteristics such as gender, race, ethnicity, or nationality, do not significantly impact assessment results. Two main types of assessment bias are offensiveness and unfair penalization.
Negative stereotypes and exclusive/offensive language can distract students from performing well. For example, a chemistry assessment that only includes contributions from male, American-born scientists may be offensive to students from international backgrounds and different gender identities. Diversity in assessment material better represents the classroom environment and can increase motivation and inclusivity for students from different backgrounds.
Assessment items that require previous knowledge or experiences that aren’t equally accessible to all students will give students from certain backgrounds an advantage. For example, an assessment on the chemistry of cheese making that requires students to know the process of making cheese from milk may favor students who come from rural backgrounds over urban backgrounds.
Clarity of Language
Clear language on assessments is a contributing factor to assessment validity. If students do not understand what the question is asking, then the question cannot accurately test the intended material.
Assessment terms for testing lower-level cognitive skills (Bloom’s taxonomy) include list, state, name, describe, and explain.
Assessment terms for testing higher-level cognitive skills (Bloom’s taxonomy) include compare/contrast, analyze, review, design, and construct.
Clear language can be improved by thinking about the purpose of each question and anticipating how the question could be misinterpreted.
Appropriate Level
The appropriate level for an assessment is determined by student age, course material, and environmental factors. Testing at the appropriate level contributes to assessment validity. If the assessment level is too high or too low it will not produce results that reflect true student knowledge of the intended subject.
Appropriate reading level refers to the assessment language that is not related to subject content.
- For example, a 9th-grade science assessment that uses the vocabulary of a college-level English class may distract students from the intended assessment material, increase test anxiety, lead to longer completion times, and increase the number of misinterpreted questions.
Appropriate content level refers to the detail and scope of subject content.
- For example, both a 5th grade and a 10th-grade class learn about atomic structure, but the assessment material for the 10th-grade class would not be appropriate for the 5th-grade class and vice versa.
ELL Students
English language learners are students who come from homes or backgrounds where English is not the primary language of communication. ELL students may require extra support or modified teaching methods depending on their level of fluency.
The following list suggests some strategies for making a science classroom more inclusive for ELL students.
- Provide students with key terms and definitions from the unit.
- Incorporate visual aids, including color-coded diagrams, graphs, and flow charts with readings.
- Turn on closed captioning for video clips and pause frequently.
- Provide students with a syllabus, outline, and/or study guides for the course.
- Provide clear procedures and instructions for tasks.
- Practice consistent routines in the classroom.
- Incorporate hands-on activities to supplement readings and lectures.
- Include pictures with key terms and new vocabulary.
- Refer to the root meaning of scientific terms, since Greek and Latin roots are shared across many languages.
- Display the most commonly used terms on a “word wall” with pictures for quick referencing.
- Provide sentence stems (e.g., “Sodium hydroxide is a base because it has …”) to help students who may get stuck on phrasing.
And that’s some basic information about the TExES Chemistry 7-12 test.
