FTCE Middle Grades 5-9 Competencies
Overview
The exam has nine competencies:
- Matter (14%)
- Forces and Motion (13%)
- Energy (12%)
- Earth Science (13%)
- Space Science (6%)
- Life Science (14%)
- Effects of Factors on the Environment (10%)
- Science Learning Environment (5%)
- Scientific Process and Inquiry (13%)
So, let’s talk about Matter first.
Matter

This competency includes about 17 multiple-choice questions which make up
about 14% of the entire exam.
These questions test your knowledge of the matter, its characteristics, and how it behaves.
Let’s talk about some concepts that you will more than likely see on the test.
Components of an Atom A common topic tested in the matter section is atoms. Atoms are the basis of chemistry because they are the foundation of life. All matter is made up of atoms. Atoms are the smallest units that make you, me, and everything else you see (and don’t see) in the world.
Three Basic Parts:
Although they are tiny building blocks, atoms are a combo of three different parts that you can remember as PEN. Just as an author crafts a story with a pen, matter is created using an atom’s pen: p roton, e lectron, and n eutron.
Although they are at opposite ends of the word ‘pen’, the protons and neutrons actually work together inside an atom’s center (the nucleus).
Although they are wrapped up together, the protons and neutrons are not the same. The proton is positively charged (p = positive), while the neutron carries no charge at all (n = neutral).
Circling the protons and neutrons like clouds of negativity, the negatively charged electrons are not found inside the nucleus. Electrons are much smaller than the other two parts of an atom but are attracted to the nucleus by the protons’ positive vibes.
Why is the ‘PEN’ So Important?
The role of a proton, electron, and neutron does not change in different types of matter. However, it is the number of each of these parts found inside a particular atom that makes a thing a thing. See the example below:
Gold and argon are both elements found on the Periodic Table. Elements are substances that cannot be broken down into other substances. There are 92 naturally occurring elements.
Gold has an atomic mass of 196.967 atomic mass units. It contains the following:
Protons- 79
Electrons- 79
Neutrons- 118
Argon has an atomic mass of 39.948 atomic mass units. It contains the following:
Protons- 18
Electrons- 18
Neutrons- 22
The masses of both of these elements are very different. The protons, electrons, and neutrons contained in their individual atoms vary, as well. But do you see a pattern here? Although it isn’t true every time, the number of protons inside an atom usually equals the number of electrons. Wanting to find an element’s atomic number? Look at how many electrons and protons it has.
On the periodic table, you will find gold in spot 79 and Argon in the 13th block.
Gas Laws A gas is one of the four fundamental states of matter, along with solids, liquids, and plasma.
In the 18th century, scientists began to experiment with different gases and realized that there is a connection between the following: pressure, temperature, and volume.
Through experiments, these early science gurus realized that because the molecules of all gases are spaced far apart, they act in a very predictable way. This led to the creation of three different gas laws:
- Boyle’s Law
- Charles’ Law
- Avogadro’s Law
Boyle’s Law in Scientific Terms:
If a certain amount of ideal gas is kept at a set temperature, then the pressure and volume of the gas are inversely proportional.
In symbols, the law is represented as PV=k.
What This Really Means:
Boyle’s Law describes how when the pressure of a gas increases (doubles), the volume of the gas will decrease by half.
Real World Example of Boyle’s Law: The Balloon Experiment
Trap a small amount of air in a balloon and then tie the end with a string. Place the balloon inside of a 50 ml syringe.
Now place the bulb inside the syringe without squeezing the piston. The results: the balloon remains the same as the air escapes from the front (atmospheric pressure stays the same).
Next, close the outlet of the syringe and squeeze the piston. What do you think will happen then? The balloon becomes smaller as the pressure increases.
Charles’ Law in Scientific Terms:
When temperature increases, the volume of a gas increases, as well and at the same rate.
What This Really Means:
Even though you can’t see oxygen, helium, or any other gas, they are still matter and contain atoms. As a gas begins to heat up, its atoms start moving around more quickly. This causes its volume, or the space it takes up, to grow, as well. As it cools, the atoms will slow down and the volume will go back down.
Real World Example of Charles’ Law: The Shrinking Balloon
Maggie, an event planner, was filling up helium balloons for a birthday party. The supply room where the helium tank was kept was very hot. After filling the balloons fully, Maggie tied each one with string and took them into the room where the party would take place. After hanging up some decorations, she turned on the air conditioner. When Maggie returned with the cake a few hours later, she was shocked to find that the balloons looked like they had lost some of their air.
Maggie took the shriveled balloons back into the storeroom so that she could refill them. To her surprise, the balloons appeared to be full of helium again! Were her eyes playing tricks on her? No, Charles’ Law was at play.
Avogadro’s Law in Scientific Terms:
When at the same temperature, equal volumes of gases and pressure contain equal numbers of molecules.
What This Really Means:
This gas law explains that the number of molecules a gas has doesn’t depend on itself (what gas it is) but instead, three other forces: temperature, volume, and pressure.
Real World Example of Avogadro’s Law:
Think of four gases that you know: nitrogen, oxygen, carbon, dioxide, helium, etc. Now imagine yourself placing equal amounts (volume) of each of these gases in separate pressurized containers and leaving them in the same room. Avogadro’s Law holds that because they are under the exact same conditions where temperature, volume, and pressure are concerned, they will have the exact number of molecules when measured.
Forces and Motion

This competency includes about 16 multiple-choice questions which make up
about 13% of the entire exam.
These questions test your knowledge of physics and how force (a push or a pull) can affect an object.
Here are some concepts you should know.
Newton’s Laws of Motion Physics is the branch of natural science focused on matter and energy. A special subset of physics deals with force and motion. Most of what we know about pushes and pulls (forces) and how they move scientifically (motion) was discovered over 300 years ago by a scientist named Sir Isaac Newton. The creator of calculus, Newton, used the work of Galileo and Aristotle and added to it with his own studies of optics, advanced math, and astronomy. The theories he came up with in 1686 are the foundation of physics and are explained in detail below.
Newton’s First Law in Scientific Terms:
Every object in a state of uniform motion tends to remain in that state of motion unless an external force is applied to it.
What This Really Means:
The first law explains that any object moving at a certain speed or in a certain direction will continue on that same path until something stops it. This ‘something’ could be a person, another object, or a force like gravity or friction.
Real World Example of Newton’s First Law
A good example of Newton’s first law involves astronauts in space. Because
the force of gravity isn’t found in outer space, an astronaut (or any other object)
floating will keep floating on and on forever. This wouldn’t change unless they floated by a planet and that planet’s gravity started to pull.
Things are different on earth. Imagine yourself kicking a ball in a straight line along a grassy path. It will continue for a while. But eventually, it will start to slow and then come to a stop as a force (i.e. friction, gravity, an object in its path) acts upon it. This is Newton’s first law in action.
Newton’s Second Law in Scientific Terms:
The relationship between an object’s mass m, its acceleration a, and the applied force F,
is F = ma. Acceleration and force are vectors; in this law, the direction of the force vector is the same as the direction of the acceleration vector.
What This Really Means:
Newton’s Second Law states that the force needed to move an object is related to the mass of the object. Let’s think about that ball we kicked again. Newton’s second law suggests that the harder you kick that ball, the farther it will go. If you pluck a marble lightly, it will go a short distance. If you were to use more force, it would travel a greater distance.
It also suggests that if you use the same amounts of force on two objects with different masses, the distance they travel will vary because of a difference in acceleration.
Real World Example of Newton’s Second Law:
At this point, you’re probably tired of kicking things, so let’s take a different approach. Imagine yourself in a room with two tables: one small, one large.
A friend that is about the same size and height as you comes and stands beside you. Using the exact same amount of force, you each push one of the tables to the other side of the room. Who will get there first?
Well, the answer depends on who is pushing the small table. The small table will have a greater acceleration, so it will reach its destination faster.
Newton’s Third Law in Scientific Terms:
For every action, there is an equal and opposite reaction.
What This Really Means:
This one is straightforward. Forces are found in pairs. When a force is being used, there is always an opposite (and equal) force pushing or pulling back.
Have you ever hit something really hard? Maybe you slapped a table, hit a punching bag, or bumped your elbow into a table with a lot of force. That pain you felt is an example of Newton’s third law. Just as you exerted a force (a push) on the object you hit, the object also applied an equal force.
Real World Example of Newton’s First Law: Playing With Skates
A science teacher could demonstrate Newton’s third law through this example.
If two students are standing in front of each other wearing skates and one reaches out and pushes the other lightly, what will happen? If you said, “they will both move away from each other,” then you understand the third law of motion.
Energy

This competency includes about 14 multiple-choice questions which make up
about 12% of the entire exam.
These questions test your knowledge of energy, its characteristics, and the way it behaves under various circumstances.
The following concepts may pop up on the test.
Thermal Energy Transfer One of the basic principles of energy is that it is neither created nor destroyed, only transferred.
There are three basic ways that thermal (heat) energy is moved from place to place. They are:
- Conduction
- Convection
- Radiation
Heat Transfers Explained
Although heat and temperature are not the same things, they are closely related.
In fact, a good definition for the word heat is–
Energy that can be transferred from one object to another because of a difference in temperature (how hot or cold something is).
Conduction
Sometimes the transferring of thermal energy happens when two objects come in
close contact. This type of shift is known as conduction and happens by the collision of molecules as the heat flows from the warmer object to the cooler object.
It is important to understand that warmer molecules move a lot faster than cooler ones. So as these fast-moving ‘hot’ molecules crash into the slower (cooler) ones, they leave some of their energy behind. The transfer only stops when the temperatures of both are equal.
Conduction: Real World Examples
- The hood of a car becomes warm once the engine is turned on.
- A cool ceramic mug will become hot once coffee is poured into it.
- Heat from your hand can melt a solid ice cube if you hold it.
Not all objects are good conductors. Solids are better at transferring heat through
conduction than liquids. Liquids are better conductors than gasses. Metals are
better than plastics, and the list goes on and on. Still, conduction is a common way that heat is transferred through objects.
Convection
Convection is the second type of thermal energy transfer. It also requires contact for the transfer to occur. Just as conduction is more common with solids, convection is the more likely way of energy trade happening with liquids and gasses.
Using a circular pattern of heating, warmer areas of liquids and gasses going through the convection process rise up to cooler spots. The cooler parts of the liquid/gas then take the place of the warmer portion. This dance continues until the convection process is complete.
Convection: Real World Examples
- When water boils, heat moves from the eye of the stove to the pot and then to the water at the bottom of the pot. This water moves up and begins to circulate.
- A heater inside of a hot air balloon heats the air and so the air moves upward, causing the balloon to rise, as well.
- Steam billows from the top of a hot cup of tea; the hot energy rises up into the cooler air.
Radiation
The third form of thermal energy transfer differs from the first two ways of transferring heat in that contact between the two sources is not needed. Radiation transports energy through electromagnetic waves traveling at the speed of light, so ‘touching’ is not required. As these waves come in contact with particles, they cause them to move. As the molecules speed up, they also heat up.
Radiation: Real World Examples
- Light from the sun hits your skin and causes you to feel warm.
- Microwaves from a microwave oven come in contact with food and heat it.
Earth Science

This competency includes about 16 multiple-choice questions which make up
about 13% of the entire exam.
These questions test your knowledge of the surface of the earth, geological processes, as well as the atmosphere and its conditions.
Let’s talk about some concepts that you will more than likely see on the test.
Law of Superposition Geology is a branch of science that deals with not only the earth’s physical structure but also the processes that act on it. Just like chemistry and physics, geology has basic laws or rules that help us understand the way the earth works. One set, known as Steno’s laws, are used to explain rocks and their layers.
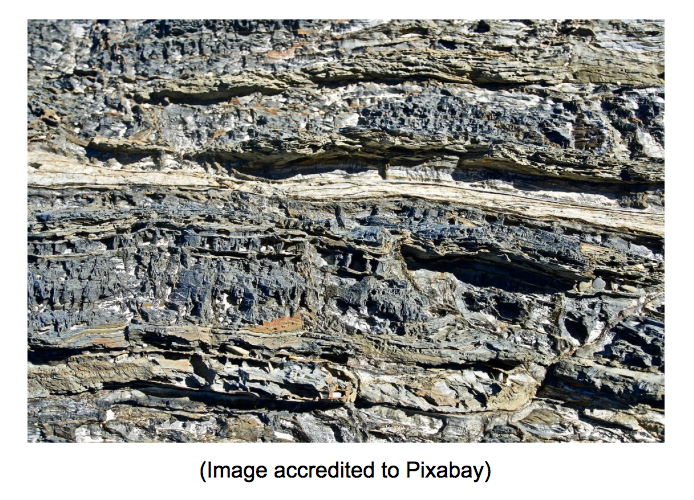
The first of Steno’s Laws of Stratigraphy is called the Law of Superposition. To understand this law better, take a look at the picture above. The layers of the sedimentary rock pictured here may look alike, but they are not the same age. This is because of the way that sedimentary rocks are made.
Sedimentary rocks are formed when weathering (ice, wind, etc.) breaks down rock and then piles it on top over and over again. As this goes on for thousands, even millions of years, new rock is formed.
The Law of Superposition states the obvious: the younger rock will be on top and the oldest layer on the bottom.
Florida’s Geology: The Formation of Florida Most people know that Florida is a peninsula because it is covered by water on three sides. But did you know that the Sunshine State is also a limestone plateau formed out of the sea millions of years ago?
What you see of the state of Florida now is just a small part of what is called the Florida Plateau or the Florida Platform . A plateau is an area of relatively level high ground, but not all of Florida’s plateau is above water. In fact, two-thirds of the platform is buried beneath the Atlantic Ocean and the Gulf of Mexico . For millions of years, the entire state was submerged.
But over time, the land began to take shape thanks to all of the small creatures that lived and died in the sea where Florida now sits. As thousands and then millions of years passed, the stacked remains of these animals were fossilized and classified by the warm sea water. The result? Limestone rock and the raised area of land that we call home.
In central Florida, this limestone is called the Ocala Group . Sitting on top is a layer of clay known as the Hawthorne Formation . The sand and clay found at this level were deposited millions of years ago as the Appalachian Mountain belt weathered and eroded. Parts of this formation have karst features –including caves, sinkholes, and shallow basins that sometimes fill with water forming lakes or ponds.
Another important geographic feature that is unique to Florida: the Ocala Uplift . This area stretches down central Florida, running parallel with I-75.
The lower part of the state has a rich geological history, as well as the Florida Keys which were formed during the last ice age over 100,000 years ago. During this time, the sea level dropped and the exposed fossils became what is now a chain of limestone islands. Two dominate rock formations, the Key Largo Limestone and the Miami Oolite, were formed during this time, as well.
Space Science
This competency includes about 7 multiple-choice questions which make up
about 6% of the entire exam.
These questions test your knowledge of outer space and the components contained in our solar system. It also tests on space explorations and technological advancements.
Here are some space-related concepts you need to know.
Stars Interesting celestial bodies, stars are huge spheres of gas that are held together by one force: their own gravity. Our own galaxy, the Milky Way, contains billions of these plasma (gas) giants and many other galaxies contain them, as well. The sun, a G2 yellow dwarf star, is the closest to earth.
Astronomers classify stars into seven classes
based on their properties , especially mass and temperature. Other properties include:
- Brightness-A combination of a star’s luminosity and distance.
- Color-The color of a star is based on its surface temperature.
- Chemical composition-What the star is made of.
- Distance-How far away a star is from a particular point.
- Luminosity-The total amount of energy emitted per unit of time.
- Size (radius)-How big the star measures.
Stars are born out of gas and dust. This mixture, known as nebulae, is the basis of the internal nuclear reactions that cause stars to burn brightly for very long periods of time.
Like animals and plants, stars have life cycles which we refer to as an evolutionary pattern . The life pattern of a star and how long it ‘lives’ is dependent on its mass (size).
Generally, the larger a star is, the shorter its life cycle will be. This is because the bigger a star is, the more quickly it will use its fuel (hydrogen).
For more information on the life cycle of a star, check out this resource: https://imagine.gsfc.nasa.gov/educators/lessons/xray_spectra/background-lifecycles.html
Life Science
This competency includes about 17 multiple-choice questions which make up
about 14% of the entire exam.
These questions test your knowledge of living organisms and their major structures and functions. It also covers DNA and genetics as related to biology.
Check out these important concepts.
Prokaryotes versus Eukaryotes The first step to understanding biology and other branches of life science is having a general idea of what living organisms are made of.
Anything living, from microscopic bacteria to the biggest animal to have ever walked the earth, is made of the same thing: one or more cells. Cells, the basic units of all life form, contain genetic material (DNA) that makes us who we are. Cells come in many different shapes and sizes, but there are two main types: prokaryotic and eukaryotic.
Two Categories:
An organism will fall into one of two categories depending on what kind of cells it contains. The first, prokaryotes , have been around for 3.8 billion years. These organisms have very simplistic cells. Prokaryotic cells have some of the parts that more advanced cells have (i.e. a cell membrane), but they are missing a cell center (nucleus) and specialized parts like mitochondria. Because they have no nucleus, the DNA of prokaryotic cells is left floating around.
Although they evolved long ago and aren’t very complex creatures, prokaryotes aren’t extinct. Just one cup full of dirt contains thousands of different species of bacteria, examples of prokaryotic-celled organisms. Prokaryotic-celled organisms are never multicellular, and the DNA in them is haploid, single, not paired.
The second grouping, eukaryotes , include plants, animals, and fungi. Eukaryotes can be unicellular, but most of the time, they have more than one cell. Eukaryotic cells are equipped with organelles surrounded by membranes that prokaryotes are missing. This includes a nucleus surrounded by a cell membrane.
Eukaryotes not only differ in their cell makeup but also in the way that they reproduce. Prokaryotes reproduce through binary fusion. Mitosis and Meiosis take place with eukaryotes. One final difference between the two is size; eukaryotes tend to be much larger.
Photosynthesis Plants are similar to animals in many ways. After all, they are both living organisms, multi-celled eukaryotes, and require energy to live. But plants and animals differ in one major way: where they get their energy from.
To live, plants need just three basic things: sunlight, water, and carbon dioxide. Unlike animals, a food source isn’t a requirement. This is because plants make their own food through a process known as photosynthesis. This makes them autotrophs. See below for the process used by plants to make their food:
Photosynthesis is the way that plants capture sunlight and use it to convert carbon dioxide and water into a chemical energy source. A key part of this food-making operation involves chloroplasts. These egg-shaped globs are only found inside plant cells. They act like factories, taking sunlight and turning it into energy. They also store chlorophyll and green pigment that gives green plants their color and help absorb carbon dioxide during light-dependent reactions. We will talk more about that process below:
Photosynthesis can be separated into two parts: light reactions and dark reactions. Like other parts of science and math, photosynthesis has an equation.
Water + Sunlight + Carbon Dioxide —> Oxygen and Food (Glucose)
- Sunlight reaches the chloroplasts, exciting the electrons inside the chlorophyll.
- Once they are activated, the electrons leave the chlorophyll.
- Any electron that left the chlorophyll must be replaced. The plant does this by splitting a water molecule and using what is inside (water and hydrogen ions.)
- The leftover electrons undergo a chemical process and make two energy-carrier molecules called ATP and NADPH.
This ends the sunlight-dependent portions of the process and the Calvin Cycle begins.
- The leaves of the plant begin to absorb carbon dioxide through holes aand thenbinds to a simple sugar, RuBP.
- Another chemical process takes place: the carrier molecules, RuBP, and carbon dioxide gets combined to create one molecule of glucose AKA plant food.
Effects of Factors on the Environment

This competency includes about 12 multiple-choice questions which make up
about 10% of the entire exam.
These questions test your knowledge of biogeochemical cycles, environmental factors, ecosystems, and how organisms survive.
Here are some concepts you definitely need to know.
Homeostasis Our bodies are made of certain parts. We start out as cells which make tissue,
which form organs, which work together to create an organ system, and then an organism (living being).
Whether we are talking about humans or some other group of animals, survival of the cells is the ultimate goal. Environmental scientists have a special term for an organism’s ability to maintain equilibrium (balance) either internally or in its environment. This is called homeostasis .
Internal Homeostasis: What is It?
Every day, we push our bodies past their boundary points. For example, if you run a couple of miles or work out at the gym for a long time, you will bring your heart rate up past its resting point. Balance disturbance can also happen when an illness takes over the body and raises body temperature or causes sweating. But our bodies, made for cell survival, have a way of bringing our systems back to order using triggers and cues within negative feedback loops. This is homeostasis at work.
Real World Examples:
One example of how homeostasis takes place every day involves the digestive system. This system’s job is to break down food and use it to fuel our bodies. Imagine you eat a piece of bread. As it enters your mouth, homeostasis begins, bringing the ph balance of your saliva to an acidic level. Your food begins to break down and your body is at a state of equilibrium. Your cells continue on.
But can you think of a situation when an organism’s body can no longer self-regulate?
A very common example of off-balance homeostasis happens when the endocrine system has trouble balancing blood sugar levels. We know this imbalance as diabetes. Thankfully, this disorder can be regulated through glucose level monitoring and replacement of insulin. If modern medicine didn’t have a solution, the survival rate for people with diabetes would go down significantly.
World Biomes: Major Life Zones The earth is made up of several distinct biomes, or very large communities in which plants and animals live. Biomes are based on geographic area and include many different ecosystems. Scientists have divided these biotic communities up based on several factors like climate, soil, and the amount of sunlight. Biome boundaries tend to be fluid and there are some transitional areas on earth that may seem to fall into more than one category. But in theory, every biological community can be found in one of the following:
- Tropical rainforest
- Temperate forest
- Tundra
- Taiga
- Grassland
- Savannah
- Freshwater
- Marine
- Ice
Examining a Biome: The Taiga
The Taiga is the largest land (terrestrial) biome on earth. It is also known as the Boreal or Coniferous Forest and runs horizontally from Alaska, across all of Canada, and most of Russia. It takes 17% of all the Earth’s land surface.
Life in most of the Taiga can be summed up with just three words: cold, isolated, and difficult. The climate is frigid, food is scarce, and it takes a special type of plant or animal to survive there. Living in the Taiga is very different than spending your days in a grassland biome like the Savannah or a tropical rainforest.
There are really just two main seasons in the Taiga. The summers, rainy and humid, vary greatly from the winters which can bring temperatures as low as -65 degrees Fahrenheit. Because of the extreme weather there, not many plants and animals make the Taiga their home. However, there are some that have adapted to this harsh biome. They include:
- Coniferous trees, mosses, and shrubs
- Owls, snow hares, and bears
- Deer, eagles, and river otters
The plants and animals above all have adaptations that make life possible in the Taiga. Coniferous trees, also known as evergreens, have long waxy needles that protect them from freezing. The snow hare’s white fur is warm and blends in with the mounds of snow that cover the ground. Without these adaptations, survival would not be possible.
Science Learning Environment
This competency includes about 6 multiple-choice questions which make up
about 5% of the entire exam.
These questions test your knowledge of science instruction and how it should take place.
Let’s talk about some concepts that you will more than likely see on the test.
Strategies for Teaching Scientific Inquiry Long gone are the days where science teachers simply opened a textbook, read from its pages, and then assigned some vocabulary definitions for students to memorize. Now, teachers are expected to help students formulate questions about the world and figure out the answers, not just repeat information presented.
A cornerstone of excellent teaching, inquiry-based activities, have been found to be much more effective tools, especially for teaching science. Inquiry-based learning shifts some of the responsibility from teacher to student and allows them to take a real interest in what they are learning.
What Inquiry-Based Learning Looks Like
- Phenomena instead of topics.Think back to your days in middle school. If you were studying plate tectonics, what might that lesson have looked like? Your teacher probably would have started the class period by saying something like, “Today, class, we are going to learn about plate tectonics” and then gave a lecture, showed pictures or a video, and assigned an activity or experiment. For inquiry-based learning, you must flip this mold upside down.
- Instead of focusing on a topic, inquiry lessons focus on aphenomenon, something going on in the natural world that needs to be explained. Maybe there was a huge earthquake that just happened in California or a tsunami in Japan. In an inquiry-based classroom, the teacher would use video clips, news articles, photos, and audio recordings to peak students’ interests in what is going on, but not explain. There is not a lot of initial discussions here. If you must pre-assess their prior learning through a chart or some other tool, keep it simple.
- Students, not teachers, ask the questions.After the children have had time to take in the scientific phenomenon presented, have them ask questions about what they saw. They might come up with things like “What caused the earthquake?” and “Why did the ocean water come onto the land like that?” Push them to think deeper and ask advanced questions.
- Lots and lots of research.Next, give them the chance to find the answers themselves through research and discussion. Do not give them the information! Allow them to dig for it through reading, hands-on experiments, and discussions with their peers.
- Projects and presentations.One thing that is difficult for some teachers is moving away from the brick and mortar tests that we are so used to taking and giving. But not all of science lends itself well to this kind of assessment. Instead, grade their experiments. Allow students to present in project form. Not only will they learn more as they complete these activities, but they will also have an outlet for expressing more of what they know.
Assessment Tools and Strategies The goal of all teachers regarding assessment is to provide one that is authentic. This means
it aims to measure student abilities in situations that reflect how those skills will actually be used. So, if we are teaching children to question and investigate the world around them, why would we stick to simple pencil and paper to test these skills?
Alternative tools and strategies for science include:
- STEM-Based Experiments.STEM stands for science, technology, engineering, and math. By incorporating all of these disciplines into one lesson, a teacher can help enhance students’ problem solving skills. The same is true of assessment. Instead of using multiple choice questions to determine what students learned about levees, have them build one!
- Online Resources.One of the most difficult parts of switching from textbook to inquiry-based learning is finding affordable resources. The world wide web is a perfect place to start. A quick Google search will take you to tons of links. Two favorites are Teacher Pay Teacher resources prepared by other educators (look for the free ones) and Mystery Science which offers easy, open and go lessons that walk kids step by step through the inquiry process.
- Virtual Exploration and Labs.Just like resources, experiments and labs can be expensive. Virtual field trips and labs can be a great alternative. Are you studying animal habitats but can’t afford a trip to the zoo? Look at a virtual one online. Looking for an interesting way to test students’ knowledge of genetics? There are virtual labs for this, as well!
- Out-of-the-Box Projects.Send-home projects are almost extinct, but that does not mean that project-based learning has to be absent from the classroom. The following assessments can be used as alternatives to fill-in-the-blank tests:
-
- Put together a time capsule of 5 things you’ve learned from this week’s lesson.
- Create a Powerpoint presentation that could be used for teaching this lesson to a younger student.
- Make a comic strip or brochure highlighting what you learned.
- Design a help wanted ad for a scientist who can help with a certain problem–what would his solution be.
- Work with a partner to make a trivia game that asks questions related to this week’s lesson.
- Create an artifact or museum exhibit that represents what you learned.
Scientific Process and Inquiry

This competency includes about 16 multiple-choice questions which make up
about 13% of the entire exam.
These questions test your knowledge of scientific knowledge, how it is gained, and how we can use what we find during investigations in the real world.
Take a look at these important concepts.
Scientific Method The scientific method is probably the most widely taught topic in both elementary and junior high science classes. Although science and the way we teach it evolves every day, this process for investigating natural phenomenons remains a staple in science classrooms. We use it to understand the world around us. We use it to answer questions about nature, the universe, and even ourselves.
Like with many other research methods, the steps for conducting an experiment using the scientific method are not set in stone. Here’s a general idea of what they might look like in an experiment.
- Ask a Question. Let’s say you go outside and notice that the three potted plants you bought at the nursery are starting to wilt. You wonder if adding plant food to them will help them grow.
- Gather Information. You do a Google search and find that there are two leading brands of plant food on the market: RichRoots and PlantBoost. You decide to buy both and try them out.
- Make a Hypothesis (educated guess). From the reviews, it seems that Richroots will work the best since it has a 4.5-star rating online. You hypothesize that it will help your plant grow at least one inch after five weeks.
- Experiment/Test. You decide to name the plants the following: plant A, plant B, and plant C (you weren’t having a very creative day). Plant A is the control subject. You don’t do anything to this plant. You add RichRoots to plant B and PlantBoost to plant C every Monday for four weeks. Every Friday, you measure your plants’ growth and record them on a chart.
- Analyze Your Test Results. On the final measuring day, you record your plant measurements and analyze the data. You find that plant A has grown half an inch, plant B an inch, and plant C two full inches.
- Present a Conclusion. Your hypothesis was correct in the sense that RichRoots did help your plant grow an inch. What you didn’t see coming is that PlantBoost would double that growth. You give it a 5-star rating online.
Traits of Scientists Close your eyes and imagine a scientist. What does your scientist look like? What is he or she wearing? Many science teachers ask students to draw a scientist at the beginning of the year. Although every picture varies somewhat, a majority picture men with beakers dressed in lab coats.
But a scientist is not dependent on a certain physical appearance. The definition of a scientist is someone who is studying or has expert knowledge of natural or physical sciences. What makes a great scientist isn’t cool glasses or a huge lab, it is possessing the following traits.
- Curiosity. Curiosity might have killed the cat, but it makes a scientist all the wiser. The first step of the scientific method is to notice and ask a question about something in nature. This takes a spirit of inquiry!
- Detail Oriented. One small mistake during the scientific method process can skew the results of an investigation. A good scientist is able to hone in on the data, paying attention to even the smallest of details.
- Critical Thinker. Logical, predicting, information seeking, evaluating. These are all parts of being a critical thinker–and a strong researcher.
- Patient. All answers to scientific questions are not found quickly. Think of the plant experiment above. It took 5 weeks to figure out the plant food mystery. Rushing through an experiment or not taking time when combing through data can ruin your results. For this reason, patience is a much-needed trait of any scientist.
- Open-Minded. Another thing that can be learned from our plant food experiment is that often, what we think will happen during an experiment doesn’t. Being free from bias and willing to accept the results of an inquiry, even if they are not what you expected or wanted, is vital.
And that’s some basic info about the FTCE Middle Grades General Science 5-9 exam.
