Not sure what to study, or if you are going to pass? We can help!
I'm a teacher candidate at a university/college I'd like to transition to teaching I'm a current / former teacher I'm in leadership for K-12 or Higher Ed (EPP)The Praxis 5245 exam has been retired and replaced by the Praxis 5246 exam. To prepare for the Praxis Chemistry (5246) exam use our updated Praxis 5436 practice test!
Praxis Chemistry Practice Test and Prep
Welcome to the Praxis Chemistry study guide, which includes practice questions. We’ll be introducing you to the core domains and concepts you need to know to pass this exam. This is one of the free resources we provide so you can see the high-level concepts you will find on the Praxis Chemistry Content Knowledge test to gauge how much you know.
Quick Links to Help You Navigate This Page
- Praxis Chemistry Test Information
- Praxis Chemistry Basic Principles of Matter and Energy
- Praxis Chemistry Atomic and Nuclear Structure
- Praxis Chemistry Nomenclature; Chemical Composition; Bonding and Structure
- Praxis Chemistry Chemical Reactions; Periodicity
- Praxis Chemistry Solutions and Solubility; Acid-Base Chemistry
- Praxis Chemistry Scientific Inquiry and Social Perspectives of Science
- Praxis Chemistry Scientific Procedures and Techniques
- Praxis Chemistry Practice Questions
Praxis Chemistry Test Information
 This test is intended to measure the competency of a secondary education Chemistry teacher. Its purpose is to measure the knowledge of an individual with a bachelor’s degree involving coursework associated with education and with chemistry. Of course, the intent is to gauge the ability of an individual to successfully teach chemistry at a secondary school level. Since the success of a student is largely dependent upon the value of the instruction they receive, the competency and literacy of individuals who pass this test is an essential tool for employers to gauge the ability and success of their staff and determine academic goals for their staff and students.
This test is intended to measure the competency of a secondary education Chemistry teacher. Its purpose is to measure the knowledge of an individual with a bachelor’s degree involving coursework associated with education and with chemistry. Of course, the intent is to gauge the ability of an individual to successfully teach chemistry at a secondary school level. Since the success of a student is largely dependent upon the value of the instruction they receive, the competency and literacy of individuals who pass this test is an essential tool for employers to gauge the ability and success of their staff and determine academic goals for their staff and students.
The Chemistry Praxis®️ test is strictly SR (selected-response) questions. There are approximately 125 questions on the test and you will be given 2.5 hours to complete the test.
The test is divided into seven (7) categories which are divided by content. Approximately 12% of the exam is over scientific procedures and techniques, another 12% is over atomic and nuclear structure, and another 12% is over scientific inquiry and social perspectives of science. About 14% of the test covers basic principles of matter and energy, 15% covers nomenclature, chemical composition, bonding and structure and another 15% covers solutions and solubility. Finally, a seventh component of the test makes up about 20% of the content and covers chemical reactions and periodicity.
Cost:
There is a $120 fee to take the Praxis®️ Chemistry Content Knowledge Assessment. There may be additional fees incurred if you end up needing to change the date or location of your test.
Scoring:
Your score is based on the number of questions you answer correctly. There is more than one version of the test and content varies from version to version. To make the various editions of the test comparable, raw scores are converted to a scaled score.
Check out your state’s score requirements here.
Study time:
In order to pass, you should break the test topics down into your strengths and weaknesses. Give yourself enough time to work on each topic until you feel confident with the majority of the subjects. Use the ETS Praxis®️ Study Plan to map out a study plan detailing the time you need to study each topic and the days you plan to do it. You can find that document here:
https://www.ets.org/praxis/prepare/materials/5245
From this site, select “study plan.” It is recommended you utilize this tool prior to studying to create a plan in order to achieve your study goals.

What test takers wish they would’ve known:
- You may encounter test questions that exam writers have incorporated into the test as practice questions that may or may not later be added to the test as a regular question in future exams. Do not be alarmed if you come across a question that you feel may not be worded well or doesn’t make sense. Do your best to answer it correctly, but it’s possible this is just a test question that will not count toward your score.
- Don’t spend too much time on a question you don’t know and risk losing valuable time on future questions that you would know. You risk running out of time if you allow more difficult questions to occupy too much of your time and don’t allow yourself enough time to read every question.
Information and screenshots obtained from the ETS Praxis®️
.
Praxis Chemistry Key Concepts to Know
Basic Principles of Matter and Energy; Thermodynamics
This content category has 17 selected-response questions. These questions account for 14% of the entire exam.

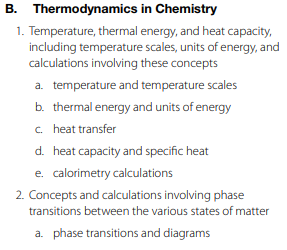

Key Concepts
Chemical and Physical Properties and Changes
Physical properties are characteristics that can be displayed without altering the chemical composition of a substance. Physical properties can be observed or displayed without changing the physical state of the substance. These can include color, density, or mass of a substance. Physical changes are also observable without altering the chemical composition of the substance, but physical changes may alter the physical state the substance exists in. Examples of physical changes include melting an ice cube, which is still water (H2O) but in a different physical state. Other examples of physical changes include the chopping of wood, burning a candle, dissolving sugar in water, or grinding metal into a powder substance.
Chemical properties involve one type of matter being changed into another type of matter. Examples of chemical properties include toxicity, flammability, solubility and acidity. Chemical changes do involve a change in chemical composition and involve a type of matter that is different than the type of matter that existed prior to the change. Iron rusting is an example of a chemical change. The combustion of a match being lit is another example of a chemical change. These changes make a substance’s ability to return to its former state impossible as the change altered the chemical composition of the substance beyond the ability to reverse the change back to its original state.
Conservation of Energy and Matter
The law of conservation of energy essentially states that within an isolated system, the total amount of energy will remain constant. Energy can not be created and it cannot be destroyed but instead, it is conserved within the system over time.
The law of conservation of mass builds off of the law of conservation of energy in that in order for the amount of energy within a system to remain constant, the mass of that system must also remain the same. If mass is added to or subtracted from the system, the energy within that system won’t remain constant. As long as the mass of the system is constant, the amount of energy within it will also remain constant.
Laws of Thermodynamics
Thermodynamics is a branch of physics that deals with the interactions between heat and energy and considers the work that is responsible for producing both. The first law of thermodynamics considers that the laws concerning the conservation of energy should also apply to thermodynamics since heat is a type of energy. So we learned just above that the law of the conservation of energy means that energy isn’t created or destroyed but rather preserved within a closed system over time. The first law of thermodynamics in an equation is the change in energy within a system, represented by “
Δ U ” , is equal to the total amount of heat transferred into the system, or “ Q ”, minus the amount of work done by the system, or “ W ”.
The second law of thermodynamics says that entropy, or the amount of disorder within an isolated system, is always increasing. Finally, the third law of thermodynamics states that as the temperature of a closed system approaches the lowest temperature possible, the entropy of that system reaches a constant value too.
Since a change in the state of a substance, or a change in the phase of a substance, is accompanied by the exchange of heat between two substances, we can conclude that the laws of thermodynamics are associated with phase changes. For instance, if you heat a solid substance, the kinetic energy of the molecules increases and energy weakens bonds between the molecules within the substance. To melt something that is in a solid state, energy has to be applied. The energy necessary for pulling off these phase changes has to come from somewhere since we know from the first law of thermodynamics that energy is conserved; it almost always comes from an external, secondary source.
Praxis Chemistry II: Atomic and Nuclear Structure
This content category has 15 selected-response questions. These questions account for 12% of the entire exam.


Key Concepts
Atomic Structure
Atoms possess a nucleus with positively charged protons, and neutrally charged, or uncharged, neutrons. Negatively charged electrons occupy the space immediately surrounding the nucleus in structured orbitals. It is important to note, so here it is again, the atomic nucleus is composed of protons and neutrons only. The number of protons, combined with the number of neutrons represents the atom’s atomic number. If that number changes, the atom being represented changes. The number of protons in an atom’s nucleus represents the atom’s atomic number and, thus, a unique element. The number of protons, in conjunction with the number of neutrons, gives you the atom’s atomic weight, which is the number of protons and neutrons in the nucleus of that atom. So, in summary:
Atomic Number: number of protons in the nucleus
Atomic Weight/Mass: number of protons + neutrons in the nucleus
If atoms contain the same number of protons in their nucleus as they do electrons orbiting the nucleus, they are considered electrically neutral. If they do not, they are considered charged. An electrically charged atom is an ion.
To get some perspective on relative sizes of subatomic particles, the diameter of the nucleus is substantially smaller than the diameter of the atom. However, a majority of the atom’s mass is contained within the nucleus. Protons and neutrons are substantially heavier than electrons. As such, electrons comprise a majority of the volume of an atom.
Electrons occupy “shells” equidistance from the nucleus, in multiple layers. Shells closest to the nucleus are lower in energy and thus allow for less electrons. Atoms fill their lower level shells first before the electrons occupy higher energy levels, further out from the nucleus. The first shell, nearest to the nucleus is labeled 1n and holds 2 electrons. The second shell is labeled 2n and holds 8 electrons. The third layer shell, 3n, holds 18 electrons. Once you reach the outermost layer of electrons, that shell is known as the valence shell. Atoms are most stable, and less reactive, when their valence shell is full. The “principal quantum number” describes the energy level of an electron within an element.
Nuclear Reactions
Nuclear fission is simply the splitting of a heavy nucleus into two lighter ones which is accompanied by the release of energy. Nuclear fusion is when two lightweight nuclei combine to form a single, heavier nucleus. This reaction is accompanied by the release of a large amount of energy. Think of “fusion” as two things fusing into one. Nuclear fission and nuclear fusion are the exact opposite of one another. Both concepts involve either the dispersion or the combination of the nucleus’ subatomic particles.
Another type of nuclear reaction is nuclear decay. This occurs when an unstable isotope transforms into another element by emitting radiation. This process involves the release of a large amount of energy in the form of radiation. Transmutation is another type of nuclear reaction that is essentially nuclear decay in reverse. While the outcome of one element being changed into another element is the same for both nuclear decay and transmutation, the process is different. In transmutation, an element is blasted with high energy radiation, which is high energy neutrons.
Essentially, nuclear reactions occur when one element spontaneously transforms into another element. They occur randomly and can take anywhere from seconds to thousands of years to occur which contributes to their unpredictable and spontaneous nature.
Electronic Energy Transitions in Atoms
Electrons can move energy levels by changing locations. In order for an electron to move to a higher energy level, it has to obtain more energy. Atoms that contain electrons that have “moved up” an energy level, are termed “excited” electrons.
Atoms may also evolve into an excited state if they absorb a photon. A photon is electromagnetic radiation or a quantum of light.
A ground state is when an electron occupies its lowest energy orbit. If that atom receives energy from an external source and becomes excited, it has the potential to move into a higher energy orbit, which is an excited electronic state.
This whole process is regulated by the laws of conservation and of matter. Since we know that energy cannot be created or destroyed, we know that if energy is necessary to excite an electron, that same amount of energy will be released when the subatomic particle returns back to its original state.
III: Nomenclature; Chemical Composition; Bonding and Structure
This content category has 19 selected-response questions. These questions account for 15% of the entire exam.


Key Concepts
Mole Concept
A mole is simply a number (can also be referred to as Avogadro’s number) that represents 6.023 x 10
23
particles of the same matter. This is obviously a large number. If you don’t grasp just how large, you might want to skip ahead to learn about scientific notation before trying to understand the mole concept. A mole represents a quantity of molecules, atoms, or units whose unit must be specified when it is used. It is defined as the quantity of a substance which contains as many “elemental entities” as does 12 grams of carbon in its innate form. So, there is 1 mole of carbon atoms in 12 grams of carbon. That translates to, there is 6.023 x 10
23
particles carbon atoms in 12 grams of carbon because “6.023 x 10
23
particles” and “1 mole” are interchangable.
Bond Types
As previously discussed, an element with an electrical charge is an ion. Ionic bonding
occurs between negatively charged ions, anions, and between positively charged ions, cations. Since we know that molecules prefer that their outer valence shell be full, it would make sense that unstable elements are always attempting to achieve a more stable electron configuration by either donating or accepting electrons. Ionic bonding is where valence electrons are lost by one atom and picked up by another. Both the atom giving up an electron and the atom receiving an electron achieve a more desirable electron configuration.
Covalent bonding
occurs when electrons are shared between two elements in order to achieve stability. Essentially, two elements “share” an electron pair. The physical properties of covalently bonded atoms are lower melting points and electric conductivity compared to atoms with ionic bonds. Ionic compounds tend to demonstrate higher melting points. Covalent bonds exist between different elements as polar bonds. However, the degree of that polarity varies. Electronegativity, or how strongly an atom attracts electrons to form a covalent bond, is used to help determine the level of polarity. The larger the difference in electronegativity between two compounds, the more the polarity. As a general guide, any difference in electronegativity below 0.4 is considered nonpolar while any value over 0.4 is considered polar. If you exceed about a 1.8 in electronegativity difference amongst the two compounds, the bond is considered ionic rather than covalent.
As the name implies, metallic bonding
holds metallic structures together. It specifically occurs between highly conductive electrons and metal ions with a positive charge. Metallic bonding is the reason metals are strong, malleable,and good conductors of heat and electricity.
Correlation with Physical Properties
The bonds that make up a substance in a particular phase are usually specific to various phases. For instance, the bonds of a solid state substance can be hydrogen bonds, which are less strong than covalent or ionic bonds. This means that a molecular solid composed of these hydrogen bonds will have a lower melting point and be relatively soft.
The boiling point of a substance is the temperature where the vapor pressure of a liquid is equal to the pressure around the liquid. This results in a change in the substance from its liquid state to a vapor state. Boiling points thus can vary based on environmental surroundings. The melting point of a substance is simply the temperature required for a solid to transform into a liquid. The temperature at which a liquid becomes a solid is its freezing point. Equilibrium vapor pressure is, as it states, the pressure at which a vapor is in equilibrium with its liquid or solid condensed phase.
IV: Chemical Reactions; Periodicity
This content category has 25 selected-response questions. These questions account for 20% of the entire exam.


So, let’s talk about some key concepts you are more than likely going to see in
this content category.
Key Concepts
Periodic Table
Elements are ordered within the periodic table by their atomic number which, as you recall, is the number of protons within the atomic nucleus of that atom. Every element has a unique atomic number and thus a unique number of protons within its nucleus. The position of an atom on the periodic table is useful in determining how their electrons are arranged. For example, row 1, or period 1 of the periodic table has electrons only in shell 1n. Row 2, or period 2 of the periodic table, has electrons in both shell 1n and 2n. Beginning with period 3, electrons begin filling shell 3n.
The column an element is found in is an indication of the element’s tendencies to react. For example, elements in column 1 have one valence electron, group 5 elements have 5 valence electrons, all the way to group 8 elements which have 8 valence electrons and, thus, a full, stable valence shell.
Elements often appear on the periodic table like this:
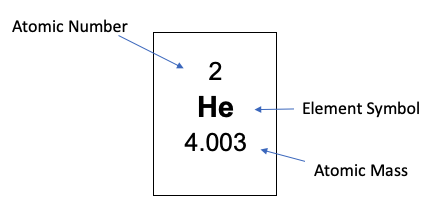
But can also appear in this format, for the same atom:
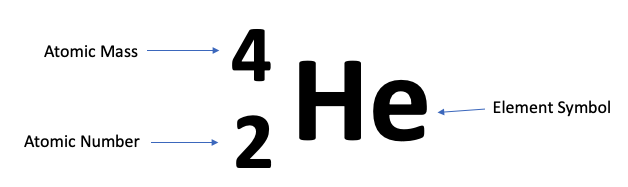
Tip:
If you get confused as to which numbers are the atomic number and the atomic mass, consider the definition of both. The smaller number will be your atomic number, since it constitutes protons alone. The larger of the two numbers will include the neutrons, in addition to the number of protons, that make up that element.
Products of Simple Reactions
Chemical reactions occur and break the bonds between molecules and even ions or atoms. In a chemical reaction, the products you begin with at the start of the reaction are the reactants, while whatever that is formed through the chemical reaction is the ending product(s). Whenever two atoms combine to form a single, more complex molecule, the result is a synthesis reaction. The exact opposite of a synthesis reaction is when a single, complex molecule undergoes a reaction to form two, more simple products is a decomposition reaction. Decomposition reactions occur regularly during the digestion process. In a combustion reaction, the oxidant, which is most often molecular oxygen, and the reductant react to produce flames.
In a single displacement reaction, one element is substituted for a different element. Often times, the oxidation of a metallic element replaces an ion in the solution. In a double replacement reaction, two compounds exchange to form two, new compounds. Single and double replacement reactions are the same, other than the number of compounds that are replaced from the reactants to the final product stage. An oxidation-reduction reaction, or a redox reaction, occurs when there is a transfer of electrons between the products and the reactants. If there is a difference in the amount of electrons from the beginning reactants to the end product, this is a good indicator that a redox reaction occurred.
Dehydration reactions occurs when water appears in the final product, which is an indication that water was lost or removed from the equation. Neutralization reactions occur when a base and an acid react to form salt and water. Be careful not to confuse this with a dehydration reaction. Technically, reactions where water are removed are dehydration reactions. But when the beginning reactants are an acid and a base, and the ending products are a salt and water, it is more specifically a neutralization reaction.
DNA and RNA
DNA, or deoxyribonucleic acid, carries the genetic information responsible for instructing cells to synthesize the proteins that make up the organism. The ability of offspring to resemble their parents occurs because they inherit their traits in the form of DNA molecules. DNA is a double stranded, long chain consisting of hydrogen bonds which bond one chain to the other. Think of DNA’s double-helix structure as a ladder. Each chain, or each side of the ladder, consists of nitrogenous bases which bind very specifically in an antiparallel fashion. On each chain is a nitrogenous base, with a phosphate backbone, which forms the outer portions of the ladder structure. Internally, sandwiched between the nitrogenous base and phosphate backbone is the signature, 5 carbon sugar, deoxyribonucleic acid.
The nitrogenous bases fall into two classes, pyrimidines and purines. The purines, adenine (A) and guanine (G) bond via hydrogen bonds only to other pyrimidines. The pyrimidines are thymine (T) and cytosine (C). Someone once told me to remember, “Go, Cowboys” and “Troy Aikman” to remember that “G” pairs with “C” and “A” pairs only with “T.”
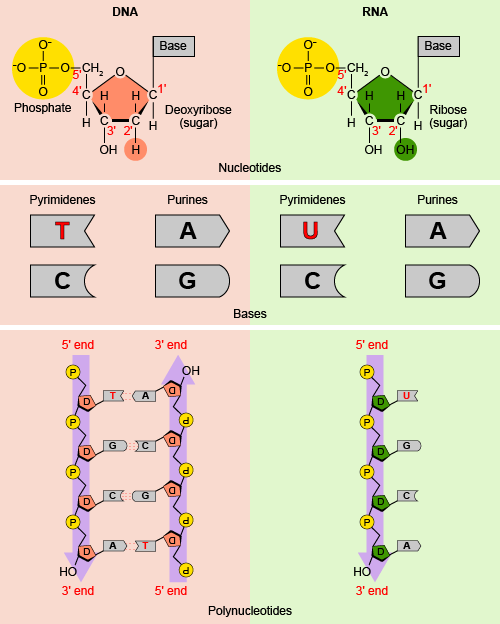
Since DNA is found in the cell nucleus and protein synthesis occurs in the cytoplasm, there has to be a way for DNA to make it out of the nucleus with a copy of the cell’s genetic instructions. RNA, or ribonucleic acid, helps to accomplish this task. RNA molecules differ structurally from DNA in that they are single stranded and much shorter molecules. Aside from the obvious component of possessing a ribonucleic acid sugar, as opposed to the deoxyribonucleic acid sugar, they also differ slightly in their nitrogenous bases. RNA also has four nitrogenous bases, but replaces the presence of thymine (T) with uracil (U) nucleotides. Just as “A” pairs with “T” in DNA, “A” pairs with “U” in RNA molecules. DNA is copied into an RNA sequence during a process called transcription, which allows for the transport of genetic material from the cell’s nucleus to the cytoplasm where protein synthesis can occur.
V: Solutions and Solubility; Acid-Base Chemistry
This content category has 19 selected-response questions. These questions account for 16% of the entire exam.


So, let’s talk about some key concepts you are more than likely going to see in
this content category.
Key Concepts
Solution Terminology
Solutions are homogeneous mixtures of at least two substances. When one of those solutions is present in a larger quantity, it is referred to as the solvent. Whatever the substance occurring in greater concentration is dissolved into, is the solute.
Solutions that contain a low amount of solute are considered to be dilute solutions. Whenever a solute’s concentration equals its solubility, that solution is considered saturated with solute. When the solute’s concentration is less than its solubility, that solution is considered unsaturated. Solutions with a solute concentration that exceeds its solubility are considered supersaturated since there is no further saturation concentration to be achieved.
Acid-Base Chemistry
Acids are electrolytes that release hydrogen ions (
H
+
). Bases are electrolytes that release ions that combine with hydrogen ions (
H
+
). Bases tend to react with acids to achieve a more neutral state. This results in the formation of water and salts. A salt is an electrolyte that forms when an acid and a base react with one another.
Arrhenius acids are substances that increase H
+
ion concentration in a solution while an Arrhenius base is a substance that increases hydroxide concentration (OH
–
).
A Bronsted-Lowry acid is a species with a tendency to donate a proton of -H
+
while a Bronsted-Lowry base tends to accept a proton.
Finally, a Lewis acid tends to accept a pair of electrons while a Lewis acid base will donate their pair of electrons.
pH Scale
Hydrogen ion concentration is measured by a pH scale. On a pH scale, which ranges from 0-14, each number is representative of a ten-fold difference in the concentration of hydrogen ions. As the hydrogen ion concentration increases, the pH value decreases. The intent of the pH scale is to measure how basic or how acidic a substance is. Thus, a pH value that is under 7 dictates an acidic solution while a solution with a pH above 7 is considered to be alkaline, or a base. Solutions that fall at a pH of 7 are considered to be neutral.
VI: Scientific Inquiry and Social Perspectives of Science
This content category has 15 selected-response questions. These questions account for 12% of the entire exam.



So, let’s talk about some key concepts you are more than likely going to see in
this content category.
Key Concepts
Scientific Knowledge
Scientific knowledge is continually evolving. Scientists are always researching, life is always evolving, and new things are being discovered every day. Generally speaking, scientific law describes a phenomenon, while the explanation of these phenomena are theories. Theories evolve with further research before becoming laws. As such, scientific knowledge continues to change in conjunction with the evolution of new research and new methodology. While at one point research may indicate that an increase in plant growth is dependent upon the addition of fertilizer, as researchers find better ways to manufacture better or different fertilizers, their products evolve too, sometimes for the better and sometimes for the worst. Not every type of research produces ideal results. Not every type of research aims to find a cure but sometimes to determine the cause.
Reproducibility is the ability of the results of an experiment to be closely replicated when the methodology of the experiment is performed again. If a particular experiment yields results that can not be reproduced again, the reliability of that study is obviously called into question . Thus, reproducibility is an important indicator of the accuracy and reliability of the methodology and results of an experiment.
Major Historical Developments
Major progress in regards to atoms was made in the late 19th century and led to significant strides in research after the end of the 19th century. English physicist J.J. Thompson’s experiment with cathode ray tubes ultimately led to discovering the presence of the subatomic particle electrons. Using an airtight glass tube with two metal electrodes, he would apply high voltage and a cathode ray would appear. Utilizing various metals for electrode composition, he noticed that a visible beam deflected away from the negative charge and toward the positive charge. Using this information, Thomson could calculate a charge-to-mass ratio of the particles and ultimately determine that the contributing particles were in fact much lighter than the atoms were. The observations lead to a rather controversial conclusion that these particles were attracted by positive charges and repelled by negative charges. This was the first indication that a negatively charged component existed as a fundamental part of the atomic structure.
In 1904, Thomson proposed a “plum pudding model” of the atom. This resembled negatively charged “plum” electrons found balanced within a positively charged “pudding.” This theory would later be disproved by a gold foil experiment and the work of Ernest Rutherford, Hans Geiger, and Ernest Mesden.
In the early 1900s, an American physicist by the name of Robert A. Millikan performed experiments where he utilized an oil atomizer to spray oil droplets into a sealed container. Here, he observed the oil landing on brass plates that were positively charged. From there, they traveled through a hole in the center of the plate, through x-rays emitted inside the container. This electrically charged the oil droplets, which finally landed on a negatively charged brass plate. He was then able to measure the charge of these oil drops and ultimately determine the charge of an electron. In conjunction with Thomson’s research, he could further determine the mass of an electron.
New Zealand Physicist Ernest Rutherford and his colleagues Ernest Marsden and Hans Geiger utilized a beam of positively charged alpha particles (α particles). They aimed beams of these α particles at a piece of gold foil and noticed that the α particles scattered. Ultimately, they found that a majority of the α particles passed through the foil and were not deflected while a very minute amount of particles were significantly deflected. What they concluded was that since the α particles passed through the foil without deflecting, they had to have traveled through a relatively vast amount of empty space. Furthermore, given the short amount of time the deflections occurred, a small positively charged body was likely centralized within the atom. This led to Rutherford’s proposal of an atomic structure with a positively charged nucleus where much of an atom’s mass is found, surrounded by electrons which yield a neutrally charged atom. It was not until the 1930s when James Chadwick pieced together that uncharged neutrons were also a part of the mass contained within the nucleus.
Around 1913, a man named Neils Bohr, a student of Rutherford’s in England, utilized both Planck’s theories of quantization and Einstein’s theory that light consists of photons with energy proportional to their frequency, and tested his own theory that an electron orbiting a nucleus would emit photons if it was found to be in a separate, orbiting plane. Bohr was able to derive the Rydberg constant, which lent support to his atomic model but accounted for only 1 electron and thus couldn’t broadly apply to all elements. Bohr was ultimately the first to discover that electrons travel in different orbits and not in a single plane. He further determined that the number of electrons varies from element to element and won the Nobel prize for his research on the atomic model.
Impact on Society and the Environment
The application of chemistry is all around us! From drinking treated water out of the facet in the morning, to taking any type of pharmaceuticals, eating any form of processed or cooked food, and putting gas into your car- you encounter chemistry at work daily. Many people accept today that chemicals like carbon dioxide, emitted from mankind’s interferences such as deforestation, and the burning of fossil fuels which contributes to global warming and the “greenhouse effect.” The decomposition of waste in landfills emits methane, another gas that contributes to global warming and environmental pollution. The maintenance of manure associated with livestock emits methane into the atmosphere while the combustion of fossil fuels and the manufacturing of commercial grade fertilizer all emit nitric oxide into the atmosphere. While chemistry research and advancements are making great strides in easing burdensome, everyday processes for us, these things don’t come without a cost. The effects of global warming may not be readily noticeable in the present tense, but they will be a problem humankind will encounter eventually. Mankind’s activities have devastating consequences on the Earth’s natural greenhouse and current global warming trends. Since the industrial revolution, humans have literally changed the composition of gases in the Earth’s atmosphere by emitting various pollutants into the environment from various processes. The presence of these gases in the atmosphere form acidic pollutants that cause acid rain.
Acid rain is an umbrella term for rainfall with unusually high acidic content or low pH levels. The term does not just apply to rain but can encompass sleet, fog, snow, hail, and other forms of precipitation. When chemical compounds are released into the air as a byproduct of human activity, they rise into the atmosphere and react with water and oxygen. Many chemical pollutants react readily with water and can then move great distances with the wind. This affects not only densely populated areas, but even areas seemingly unaffected by the pollution caused by mankind’s advancements in technology. If you think that because you don’t directly interact with one of these industries, you aren’t contributing to it, think again. Everytime exhaust comes from your vehicle, you’re emitting sulfur dioxide and nitrogen oxide into the environment. The use of electricity in your home comes from a power plant burning fossil fuels and releasing nitrogen oxide and sulfur oxide into the environment.
Chemistry makes virtually all forms of medical imaging possible. Sonograms use high-frequency sound waves to see inside human bodies. X-rays use electromagnetic radiation to create images of the human skeleton. Magnetic resonance imaging, or MRIs, use radio waves and very strong magnetic fields to create images of the human body for medical imaging. Computerized tomography, or CT images, shoot narrow beams of x-rays through the body. A specialized CT computer then takes digital x-ray detectors to create 2-dimensional images of the human body to create medical images for physicians to use for more reliable, modern diagnostic analysis.
VII: Scientific Procedures and Techniques
This content category has 15 selected-response questions. These questions account for 12% of the entire exam.

So, let’s talk about some key concepts you are more than likely going to see in
this content category.
Key Concepts
Scientific Notation
Scientific Notation is a way to express numbers that are much too large (think the mass of the Sun) or much too small (think the mass of a dust particle). Using scientific notation, we can express numbers in a way that is far easier for people to comprehend and assess the magnitude of as well as visually reduce all of the zeroes that either trail the number of precede it. When we see this many zeroes, it sometimes makes it difficult to assess just how big or how small something really is.
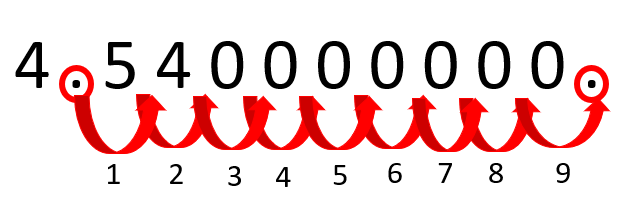
Let’s consider the age of the Earth. It is approximately 4,540,000,000 years old. That number can be converted to scientific notation and instead read that the Earth is 4.54 x 10
9
years old.

In this example, the coefficient is 4.54. The coefficient must be a number greater than 1.0 and less than 10. In this case, it is 4.54. Next, you place the decimal point in a place that makes the value of the coefficient between 1 and 10. The base is always 10, the standard base unit for scientific notation. From there, count over how many places, or 0s until you reach the end of the value you’re notating.
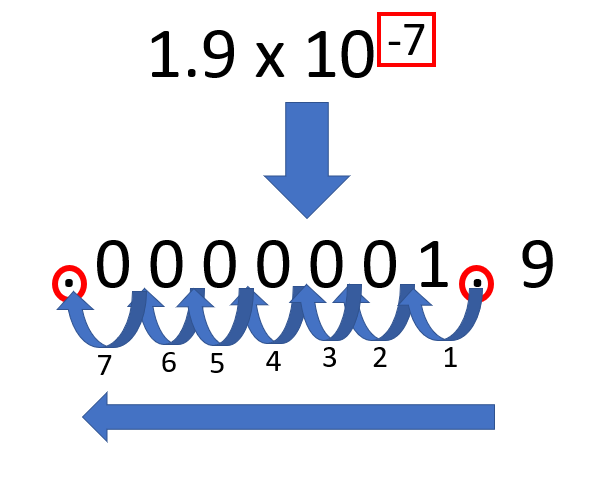
The last important thing to mention in regards to scientific notation, is when you are moving the decimal place to the right of the number, the exponent is a positive value. But when the decimal place moves to the left to convert to scientific notation, the exponent is a negative value. See the example below.
Safe Disposal
Label every item in a laboratory. If something is not labeled, do not use it. Dispose of any unlabeled items in the laboratory. Keep an up to date inventory of all chemicals in the lab. Every lab should have a procedure in place for disposing of unidentified chemicals in a lab. This process often includes a data sheet that accompanies the unidentified item. Do NOT assume you can safely dispose of an unknown chemical when it is not properly labeled, but instead follow your lab’s protocol for disposal of unknown substances.
The improper handling of sharp objects in a laboratory setting can lead to the largely preventable, transfer of infectious diseases. Use of proper sharps containers for the disposal of sharps in a laboratory setting is important. Sharps containers are at capacity when they are about ¾ of the way full. Additional sharps to be disposed of should not be forced to fit into a sharps container that is already ¾ full.
Safety Procedures
A typical laboratory is equipped with both (A) equipment to navigate an accident in the event that it happens, as well as (B) personal protective equipment (PPE) to proactively keep staff and students protected from materials they encounter on a day to day basis while doing lab work.
Some of the standard equipment you will see is a fire extinguisher and fire blanket. In the event that something does catch on fire, an extinguisher can control small flames until public safety professionals can arrive on scene to assist. The fire blanket is made of a non flammable material that can be used to put out flames on a person since a fire extinguisher is less safe on a person than a blanket. Other items in a lab setting room should include an eye wash station, or at least a faucet-mounted eyewash. In the event that someone gets any chemical in their eyes, this is a simple and very effective way to safely wash it out with water. An individual simply leans over the eyewash device and turns on the water pressure. The eyes are flushed with water for a period of 15 minutes. Often times in lab settings, the room is also equipped with a safety shower which is used if any corrosive or toxic chemical is spilled on clothing. In that event, an individual would stand under the safety shower and rinse their clothing for a period of 10-15 minutes until they can remove the clothing.
Personal protective equipment should be stocked in any lab setting to adequately protect the skin and clothing of the maximum number of individuals in the classroom at any one time. This would include disposable gloves (latex and nitrile), lab safety goggles, and lab coats or lab aprons. If there are any methods by which you heat anything in the lab, at least one pair of heavy duty, heat resistant gloves should also be kept in the lab for handling hot or potentially hot objects as disposable gloves will not suffice for protection from high temperatures.
It should be noted in the classroom setting and somewhere in the room that closed-toed shoes and fully clothed legs are required in a lab setting. Long hair should always be tied securely back. It is highly recommended that teachers have students sign off on these safety measures at the beginning of the course to verbalize understanding of these lab safety precautions and to demonstrate their agreeance with compliance.
And that’s some basic info about the exam.
Now, let’s look at a few practice questions in each area to see how these
concepts might actually appear on the real test.
Praxis Chemistry Practice Questions
Question 1
The specific heat of liquid water is 1 cal/kg°C. The specific heat of ice is half of that, at 0.5 cal/kg°C. Compared with that for 2 kg of liquid water, how much heat is needed to change the temperature of 2 kg of ice by 10°?
- Four times as much heat is needed to change the temperature of the ice.
- Twice as much heat is needed to change the temperature of the ice.
- Half as much heat is needed to change the temperature of the ice.
- One fourth as much heat is needed to change the temperature of the ice.
Correct answer: 3.
The heat needed equals the product of mass, temperature change, and specific heat. Since the specific heat is half as great for ice, only half as much heat is needed to change its temperature by the same amount.
Question 2
Which of the following correctly explains the difference between heat and thermal energy in science?
- An object has thermal energy, but heat is transferred between objects.
- An object has heat, but thermal energy is transferred between objects.
- There is no difference. Heat and thermal energy are the same thing.
- Heat is a measure of how hot an object is, whereas thermal energy is a measure of how fast the object’s molecules move.
Correct answer: 1.
The energy transferred because of a temperature difference is heat. The internal energy of an object is thermal energy.
Question 3
The specific heat of oak is 0.58 cal/g°C. How much heat is required to change the temperature of 10 grams of oak by 10°C?
- 5.8 cal
- 580 cal
- 0.58 cal
- 58 cal
Correct answer: 4.
The heat required is the product of the specific heat
c
p
, the mass,
m
, and the temperature change Δ
T
.
Q
=
mc
p
Δ
T
Q
= (10 g)(0.58 cal/g°C)(10°C) = 58 cal.
Question 4
Which of the laws of thermodynamics prohibits any process from being 100% efficient?
- the zeroth law
- the first law
- the second law
- The first and second laws when taken together prohibit 100% efficiency.
Correct answer: 3.
The second law, often called the law of entropy, prohibits 100% efficiency in any process.
Question 5
Determine the change in internal energy of a pot of eggs put on the stove and brought to a boil if 200 kJ of heat is added to the water, 80 kJ of heat escapes from the pot, and the escaping steam does 25 kJ of work vibrating and lifting the lid of the pot.
- 95 kJ
- 175 kJ
- 145 kJ
- 305 kJ
Correct answer: 1.
The first law of thermodynamics states that the change in internal energy Δ
U
of a closed system equals the heat
Q
added to the system minus the work
W
done by the system (Δ
U
=
Q
–
W
). If we assume this is a closed system, 200 kJ of heat is added and 80 kJ of heat escapes, leaving a net of 120 J of added heat. Subtract the work done by the system on the lid to obtain 95 kJ. The internal energy of the system increased by 95 kJ.
Question 6
Which of the following will give off energy if used as fuel in a fission reaction?
- helium (He)
- thorium (Th)
- beryllium (Be)
- hydrogen (H)
Correct answer: 2.
Fission is the splitting of heavy elements into lighter elements. All elements heavier than iron emit energy during fission.
Question 7
Which of the following is powered by fusion reactions?
- currently functioning nuclear power plants
- heating of Earth’s interior
- the Sun
- launching rockets for satellites
Correct answer: 3.
Deuterium (
²
H) and tritium (
³
H) fuse in the Sun to form energetic helium (
⁴
He) and neutrons.
Question 8
Select from the following list all of the problems with the planetary model of the atom.
- It did not explain why electrons do not fall into the nucleus.
- It did not model the behavior of heavier elements.
- It proposed that atoms are the smallest particles of matter.
- It did not allow for the presence of neutrons in the atom.
Correct answer: 1 and 2.
The planetary, or Bohr, model of the atom gives no explanation of why electrons remain in orbit around the nucleus. The planetary model does not correctly explain heavier elements.
Question 9
Which of the following is the best general description of radioactivity?
- The absorption of particles from space.
- An exchange of particles from one atomic nucleus to another.
- The stripping of electrons from an atom.
- A release of energy from the nucleus of an atom.
Correct answer: 4.
Radioactivity manifests itself in several different ways, but each involves a release of energy from an atomic nucleus.
Question 10
Which of the following is emitted from the nucleus during alpha decay?
- a helium nucleus
- one or more protons
- an electron
- pure energy; no protons, electrons, or neutrons
Correct answer: 1.
During alpha decay, a helium nucleus, consisting of 2 protons and 2 neutrons, is emitted from the nucleus.
Question 11
In which of the following types of bonds are electrons transferred from one atom to another?
- covalent
- ionic
- metallic
- intermolecular
Correct answer: 2.
Ionic bonds form when one atom transfers one or more electrons to another atom and the two atoms with opposite charges attract each other.
Question 12
Which of the following describes the attractions in a compound between hydrogen and a nonmetal such as fluorine?
- covalent bonds
- ionic bonds
- metallic bonds
- hydrogen bonds
Correct answer: 4.
When hydrogen bonds with a nonmetal, the hydrogen’s electron moves toward the nonmetallic atom leaving the hydrogen with a slightly positive charge and the nomental with a slightly negative charge. The attraction of these opposite charges (on the opposite ends of each molecule) bonds molecules together in what is known as a hydrogen bond.
Question 13
Which of the following will increase the rate of dissolution of a solute in a solvent?
- increased temperature
- an increase in solute mass
- increased particle size
- increased density of the solvent
Correct answer: 1 and 3.
In most liquids, particles dissolve more quickly at higher temperatures. Smaller particles dissolve more quickly than larger particles.
Question 14
How many grams of NH
₃
is required to form a 0.500 molar solution in 1.00 L of water?
- 8.52 g
- 14.0 g
- 3.02 g
- 17.0 g
Correct answer: 1.
The molarity of a 0.500 molar solution is 0.500 mol/L. Since there is 1 L of solvent, 0.500 mol of NH
₃
is required.
The molar mass of H in NH
₃
is 3 x 1.008 g/mol = 3.024 g/mol, where 1.008 g is the mass of one mole of hydrogen.
The molar mass of N is 14.007 g/mol.
The molar mass of NH
₃
is 3.024 g/mol + 14.007 g/mol = 17.041 g/mol.
Multiply the molar mass by the number of moles to determine the needed grams of NH
₃
: (17.041 g/mol)(0.500 mol) = 8.52 g.
Question 15
Which of the following is the correct name for the ionic compound BaCl
₂
?
- barium dichloride
- barium chlorate
- barium (II) chlorate
- barium chloride
Correct answer: 4.
A simple ionic compound is named with the metal first and the nonmetal ending in –
ide
.
Question 16
Which of the following represents a double replacement reaction?
- H
₂
CO
₃
→ H
₂
O + CO
₂
- NaOH + HCl → NaCl + H
₂
O
- Zn + 2HCl → ZnCl
₂
+ H
₂
- Mg + O → MgO
Correct answer: 2.
This is a double replacement reaction. The hydroxide in sodium hydroxide (NaOH) is replaced with chlorine to form salt (NaCl), and the chlorine in hydrochloric acid (HCl) is replaced with the hydroxide to form water.
Question 17
What is oxidized and what is reduced in the redox reaction H₂
+ F
₂
→ 2HF?
- H
₂
is oxidized, F
₂
is reduced.
- F
₂
is oxidized, H
₂
is reduced.
- H
₂
and F
₂
are oxidized, HF is reduced.
- HF is oxidized, H₂ and F₂ are reduced.
Correct answer: 1.
In a redox, or oxidation-reduction, reaction, one reactant gains electrons and one loses electrons. During the reaction each hydrogen atom has a single electron which is given to a fluorine atom to form an ionic compound; therefore, H
2
is oxidized (loses electrons) and fluorine is reduced (gains electrons). Check this using the oxidation numbers of H and F in HF: when hydrogen combines with a nonmetal, such as fluorine, its oxidation number is +1, meaning it has lost an electron. The oxidation number of each fluorine atom in HF is –1, meaning it has gained an electron.
Question 18
Which chemicals are the reactants and which are the products in the following combustion reaction?
CH
₄
+ 2O
₂
→ CO
₂
+ 2H
₂
O
- Reactants: CO
₂
and H
₂
O
Products: CH
₄
and O
₂
- Reactants: CH
₄
and CO
₂
Products: O
₂
and H
₂
O
- Reactants: CH
₄
and O
₂
Products: CO
₂
and H
₂
O
- Reactants: O
₂
and H
₂
O
Products: CH
₄
and CO
₂
Correct answer: 3.
The reactants, or chemicals present before the reaction, are on the left side of a chemical equation (CH
₄
and O
₂
), and the products, or chemicals present after the reaction, are on the right side of the equation.
Question 19
Which of the following best describes a buffer?
- a substance that resists a change in pH
- a substance that neutralizes acids but not bases
- a substance that neutralizes bases but not acids
- a substance that changes acids into bases
Correct answer: 1.
A buffer is the general name for any substance that resists a change in pH. A buffer solution contains both an acid and a base.
Question 20
What is the pH of a solution after it has been neutralized?
- 3
- 5
- 7
- 9
Correct answer: 3.
A neutral solution has a pH of 7.
Question 21
In the neutralization reaction HCl + NaOH → H
₂
O + NaCl, which compounds have a pH greater than 7, which have a pH less than 7, and which have a pH of 7?
- Greater than 7: NaOH
Less than 7: HCl
Equal to 7: H
₂
O and NaCl
- Greater than 7: HCl
Less than 7: NaOH
Equal to 7: H
₂
O and NaCl
- Greater than 7: NaCl
Less than 7: H
₂
O
Equal to 7: HCl and NaOH
- Greater than 7: HCl Less than 7: NaCl Equal to 7: H₂O and NaOH
Correct answer: 1.
NaOH, sodium hydroxide, has a pH greater than 7, as do all bases. HCl, hydrochloric acid, has a pH less than 7, as do all acids. H
₂
O and NaCl, water and salt, both have a neutral pH of 7.
Question 22
Which of the following depend on chemical reactions for their formation or application?
- medicines
- plastics
- combustion engines
- All of the above.
Correct answer: 4.
All of the above depend on chemical reactions.
Question 23
The pressure of an ideal gas is doubled as its volume is decreased by ⅓. What happens to the temperature during this process?
- The temperature decreases by a factor of ⅔.
- The temperature increases by a factor of 6.
- The temperature decreases by a factor of ¾.
- The temperature increases by a factor of 12.
Correct answer: 1.
The ideal gas law states that the product of pressure
P
and volume
V
equals the product of the number of moles
n
, the gas constant
R
, and the temperature T:
PV
=
nRT
. Inserting 2
P
and
V
/3 for pressure and volume indicates the temperature must decrease by a factor of ⅔ since
n
and
R
remain constant: 2
P
(
V
/3) =
nR
(2
T
/3).
Question 24
Nicole makes two solutions of salt water. For solution 1, she adds 1 tablespoon of salt to 1 cup of water and stirs until the salt has dissolved and the solution is clear. For Solution 2, she adds more than 1 tablespoons of salt to 1 cup of water, and although she stirs and stirs, the solution remains cloudy. Which of the following describes the most likely end result?
- solution 1: unsaturated; solution 2: unsaturated
- solution 1: unsaturated; solution 2: saturated
- solution 1: saturated; solution 2: saturated
- solution 1: saturated; solution 2: unsaturated
Correct answer: 2.
An unsaturated solution can hold more solute (salt in this case), whereas an unsaturated solution cannot. Solution 1 can probably hold more salt. Some of the solute in solution 2 remains undissolved, leaving the water clouded with undissolved salt.
Question 25
Which of the following is true in science?
- Scientific laws are the same as facts.
- Science is open to change.
- Scientific theories always evolve into scientific laws.
- Scientific laws and theories are the same.
Correct answer: 2.
Science is open to change as new information is discovered.